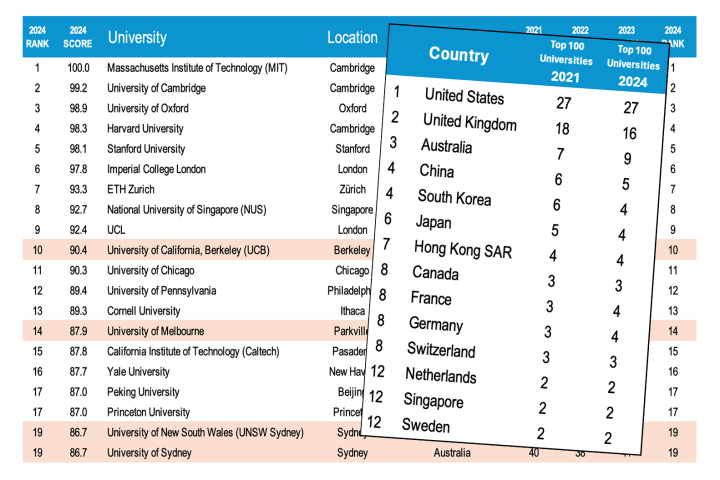
There can be little doubt that people love their mobile devices. But, by leaving them high and dry at the most inconvenient of times, this love generally doesn't extend to the batteries that power said devices. New microbatteries developed by researchers at the University of Illinois at Urbana-Champaign (UIUC) that measure just a few millimeters in size, yet are powerful enough to power a mobile phone may be more likely to inspire a little love.
A battery's ability to store and provide energy faces the fundamental limitation that its energy source is chemical in nature. Conventional battery designs are essentially a pair of plates made of different metals separated by an electrolyte that allows movement of electrons and ions between them.
This construction leads to three significant limitations that affect most batteries:
- Energy capacity is related to the physical size of the battery and the energy of the chemical reactions powering it.
These factors place remarkably tight limits on the performance of practical batteries for mobile devices.

At present, the common rechargeable battery which supplies the most energy is the lithium-ion battery, with an energy density of about 300 mWh/cc (milliwatt-hours per cubic centimeter). For comparison, burning one cubic centimeter of gasoline produces 9700 mWh/cc. The power density of such a battery, meaning the rate at which the energy can be delivered, is about 100 mW/cc.
At times, more power is more important than more energy storage. For example, powering a 100-kW electric car with conventional lithium-ion batteries would require a cubic meter of batteries weighing roughly 2.5 metric tonnes (2.75 tons). This is why so much engineering effort is going into the design of lithium-ion battery packs for electric vehicles.
When the need for power is paramount, engineers often turn to supercapacitors. These electric double-layer capacitors offer enormously fast delivery of energy (up to about 40 W/cc – hundreds of times faster than conventional lithium-ion batteries), but suffer from a rather small energy capacity (only a few mW-hr/cc).
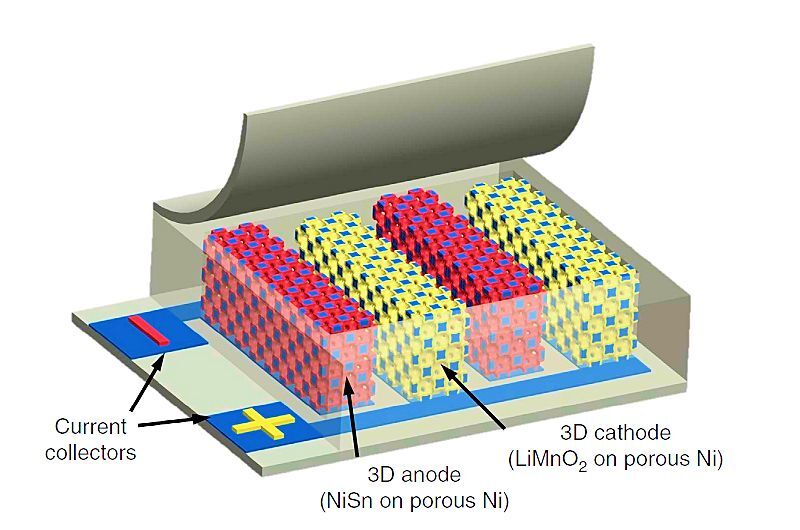
William King's team at the University of Illinois at Urbana-Champaign sought to achieve a more favorable balance between energy and power density. To provide good energy density they used a lithium-ion chemistry for their batteries, and to obtain large power density they maximized surface area by using electrodes with a highly porous 3D structure arranged in the form of interlocking strips with a separation of about a millimeter.

With this structure, the Illinois 3D microbatteries provide more power density (up to about 100 W/cc) than even the best supercapacitors and nearly as much energy density (up to 15 mW-hr/cc) as conventional lithium-ion batteries: factors that could drive new small-scale battery applications in radio communications, medical devices, computer hardware, and compact electronics.
The team's 3D microbattery design is particularly suited to physically small circuits, for example an implanted heart pacemaker and defibrillator, where a comparatively large battery must currently be used to supply large spikes of current when needed. Such spikes could easily be delivered by a tiny 3D microbattery. In addition, the new batteries can be charged as fast as they discharge, meaning recharge times of under a minute are possible, a quality that largely offsets the limited energy storage offered by any tiny battery.
"Now we can think outside of the box," says James Pikul, lead author of the associated paper, published in Nature. "It's a new enabling technology. It's not a progressive improvement over previous technologies; it breaks the normal paradigms of energy sources. It's allowing us to do different, new things."
Source: University of Illinois