A remarkable new study has successfully used the CRISPR-Cas9 gene editing technique to edit a specific gene in mice engineered to have fragile X syndrome (FXS), a single-gene disorder often related to autism. The single gene edit in the live mice resulted in significant improvements in repetitive and obsessive behaviors, making this the first time gene editing has been used to effectively target behavioral symptoms related to autism spectrum disorder (ASD).
FXS is a genetic disorder associated with intellectual disability, seizures and exaggerated repetitive behavior. Previous studies have shown that the repetitive behaviors associated with FXS are related to a specific excitatory receptor in the brain that, when dysregulated, causes exaggerated signaling between cells.
The CRISPR technique homes in on the gene that controls that excitatory receptor, the metabotropic glutamate receptor 5 (mGluR5), and essentially disables it, dampening the excessive signaling the corresponds with repetitive behaviors. In mice treated with the new system, obsessive digging behavior was reduced by 30 percent and repetitive leaping actions dropped by 70 percent.
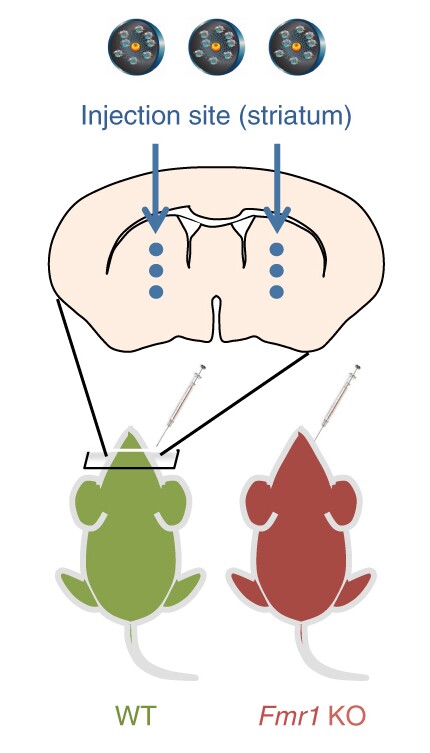
"Before this experiment, we didn't know if the mGluR5 receptor in the striatum was specifically involved in exaggerated repetitive behavior; that is an important biological finding of our study," says study leader Hye Young Lee.
An even more fascinating element of this new research was the novel CRISPR-Cas9 delivery method, pioneered by a team at the University of California, Berkeley. The most commonly used gene delivery technique for CRISPR harnesses the power of viruses to ferry the Cas9 enzyme to a targeted cell. But viral gene delivery has its limitations. As well as battling the potential for a person's immune system to develop antibodies against the virus, this delivery system makes it difficult to control how much Cas9 is ultimately delivered.
"If you inject CRISPR DNA using a virus, you can't control how much Cas9 protein and guide RNA are expressed, so injecting it via a virus has a potential problem," says Lee.
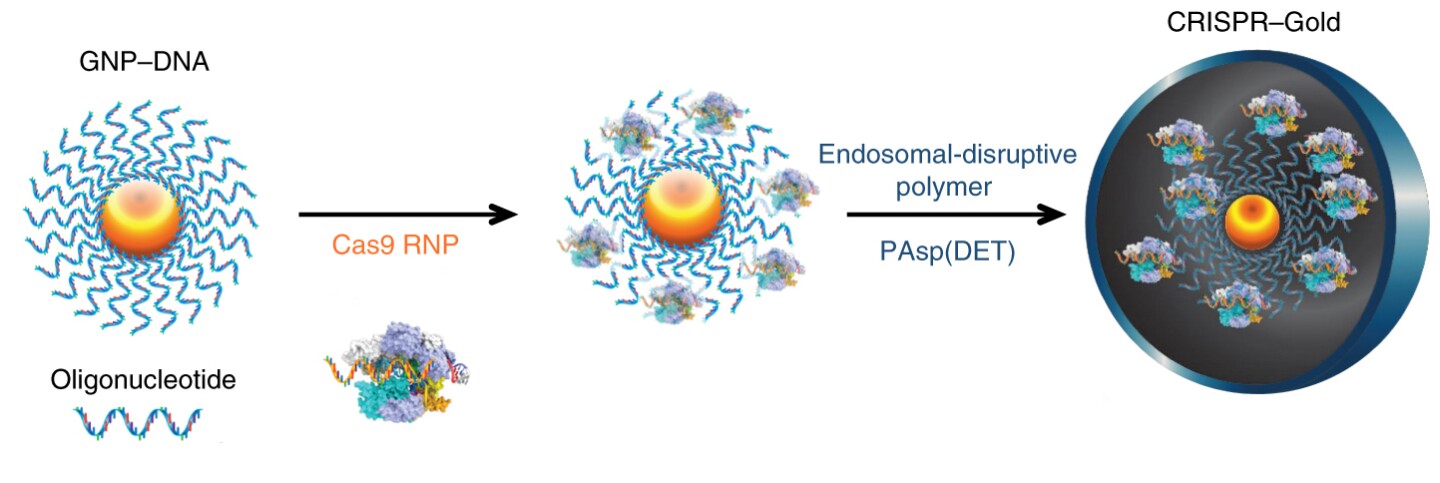
The new method utilizes gold nanoparticles to carry the CRISPR-Cas9 complex into the brain. Called CRISPR-Gold, the novel delivery system covers a gold nanoparticle with the therapeutic Cas9 molecules. A extra guide RNA is added to help the nanoparticle home in on its target gene, before the entire package is covered with a polymer that assists it in entering the correct cells.
"I think the CRISPR-Gold method is very cool because we can control the amount we wish to inject and that probably minimizes the side effects of using CRISPR, for example off-target effects," adds Lee.
This isn't the first time a non-viral CRISPR delivery method has been effectively demonstrated. For instance, last year a team from MIT successfully used a nanoparticle delivery device to silence targeted genes in mouse liver cells. However, this new study is the first evidence of CRISPR being successfully used to edit a causal gene for autism in the brain resulting in significant behavioral changes.
The next stage for the research is to work to develop more efficient CRISPR-Gold particles that can be better administered. This particular mouse study involved the particles being injected directly into the brain, a less than ideal process that couldn't easily be applied to humans. The researchers suggest that a system involving administration through the central nervous system via the spinal cord could be equally effective.
The effectiveness of this CRISPR-Gold system in accurately editing specific genes in brain cells could in the future be potentially applied to a broad assortment of different genetic diseases, as well as targeting other social interaction symptoms associated with ASD.
"CRISPR-Gold can be used to treat a variety of genetic diseases, such as Huntington's disease," says Lee. "But it's not limited to monogenic diseases; it can also be used against polygenic diseases, once we figure out the entire network of genes involved."
The new research was published in the journal Nature Biomedical Engineering.
Source: UC Berkeley






