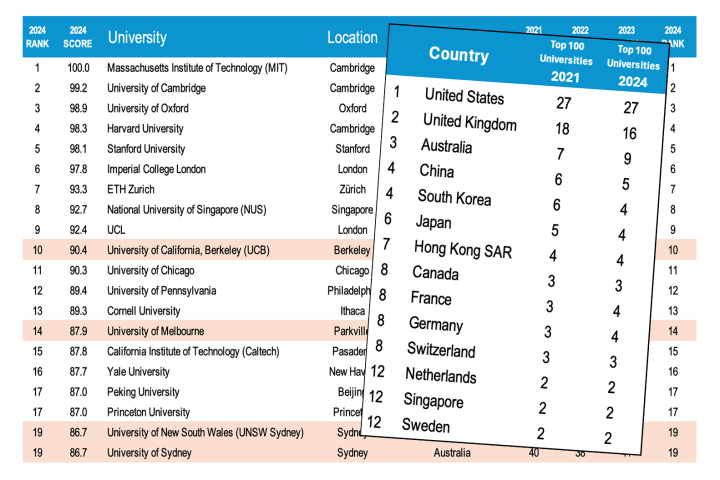
It may be a bit gross, but mucus plays a key role in our bodies. Now, an MIT team has managed to create an artificial version of the stuff, and shown that it’s an effective antimicrobial – even more so than real mucus.
Mucus coats the surfaces inside our bodies that come in contact with the outside world – the nose, mouth, throat, and gastrointestinal tract. It’s there to keep these sensitive tissues moist, but it also plays an important part in protecting us from foreign threats like bacteria.
Research has found that mucins, the proteins that make up mucus, can interfere with several vital functions in bacteria, such as their ability to communicate with each other, produce toxins, and cling to host cells. Reproducing these capabilities could make for useful antimicrobial materials that are less likely to lead to antibiotic resistance in bacteria.
“We would really like to understand what features of mucins are important for their activities, and mimic those features so that you could block virulence pathways in microbes,” says Laura Kiessling, senior author of the study.
But there’s a problem. Mucins are shaped like long, thin bottlebrushes, comprising a backbone of amino acids with sugar molecules called glycans sticking out the sides. The large size and complex shape of these proteins have made them difficult for scientists to replicate.
For the new study, the team used a new method to create mucins. Using a tungsten-based catalyst, they unfurled a carbon ring into a linear molecule that contains a carbon-carbon double bond. Importantly, this produced “cis” configurations of the double bonds, where the carbon atoms have attached chemical groups on one side. That’s in contrast to “trans” configurations where the groups appear on opposite sides.
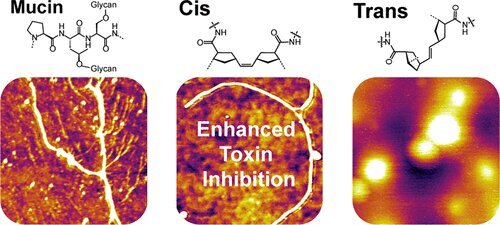
This cis configuration, the team found, was closer to the shape of natural mucins, allowing them to be more water soluble. In the next tests, the researchers exposed the synthetic mucins to toxins produced by the bacteria that causes cholera. They found that not only were the synthetic cis polymers better at capturing those toxins than the trans ones, but they even outperformed the natural mucins.
It’s a promising beginning, the team says, and they could be made even better with some tweaks to the glycans. That will be the focus of future work.
The research was published in the journal ACS Central Science.
Source: MIT