Carbon has many properties, but one word that's not usually associated with it is "stretchy." That is, not until a team of scientists from the Carnegie Institution for Science and Yanshan University developed a new form of carbon that is elastic as well as ultra-strong, lightweight, and electrically conductive, properties that lend it to a wide array of applications, from aerospace engineering to military armor.
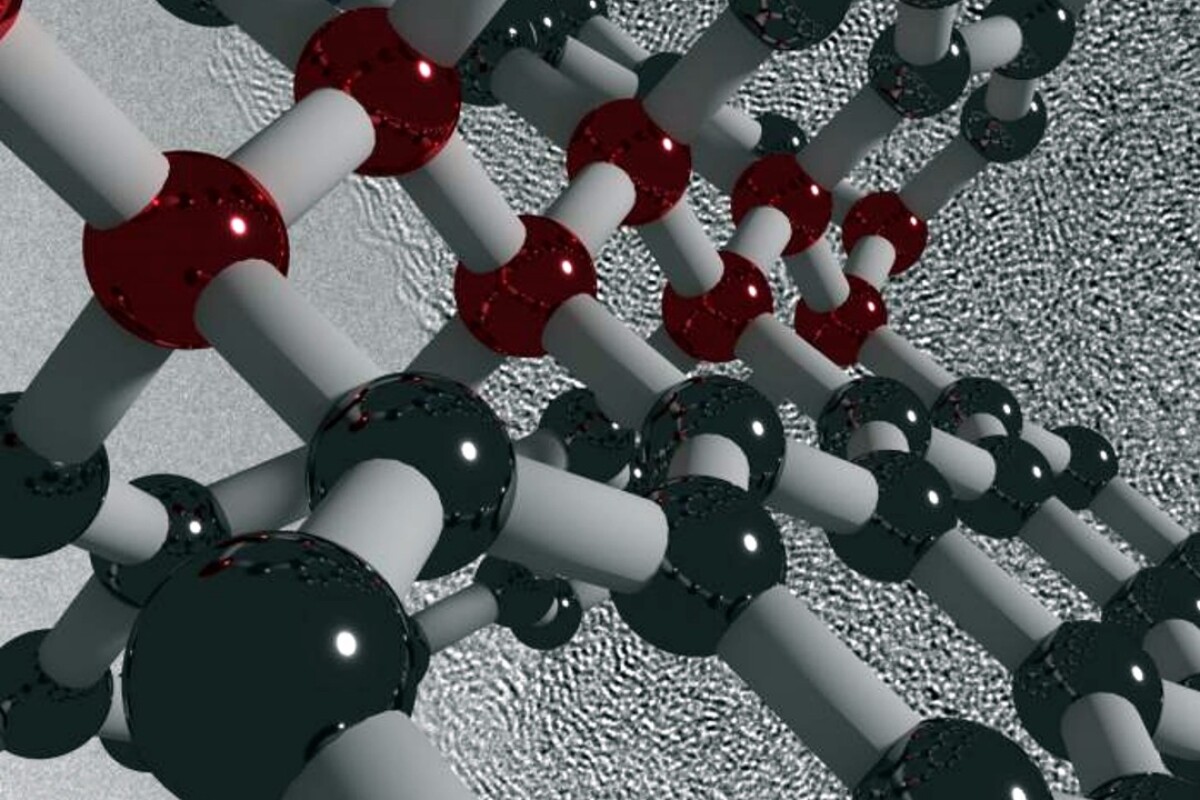
The popular idea of carbon is that it comes in two forms. Either it's an opaque, black substance like coal, or a hard, brilliant crystal like diamond. However, to chemists, it's an element that can be formed into an incredible array of substances that include the slippery flat plates of graphite molecules for pencils and lubricants, the incredibly durable crystalline matrix of the diamond for jewelry or drill bits, or exotic molecules, including the Buckminsterfullerene of 60 carbon atoms formed into an open sphere, or sheets of graphene that are 200 times as strong as the strongest steels.
The new elastic carbon is made of layers of graphene and curved molecular surfaces that are linked together with the same strong linkages found in diamonds. This makes the new substance both elastic as well as remarkably strong and light. It's produced from another exotic form of carbon called "glassy" or "vitreous" carbon. That is, carbon that isn't a true solid, but a supercooled liquid like glass. And like glass, it doesn't have a crystalline structure.
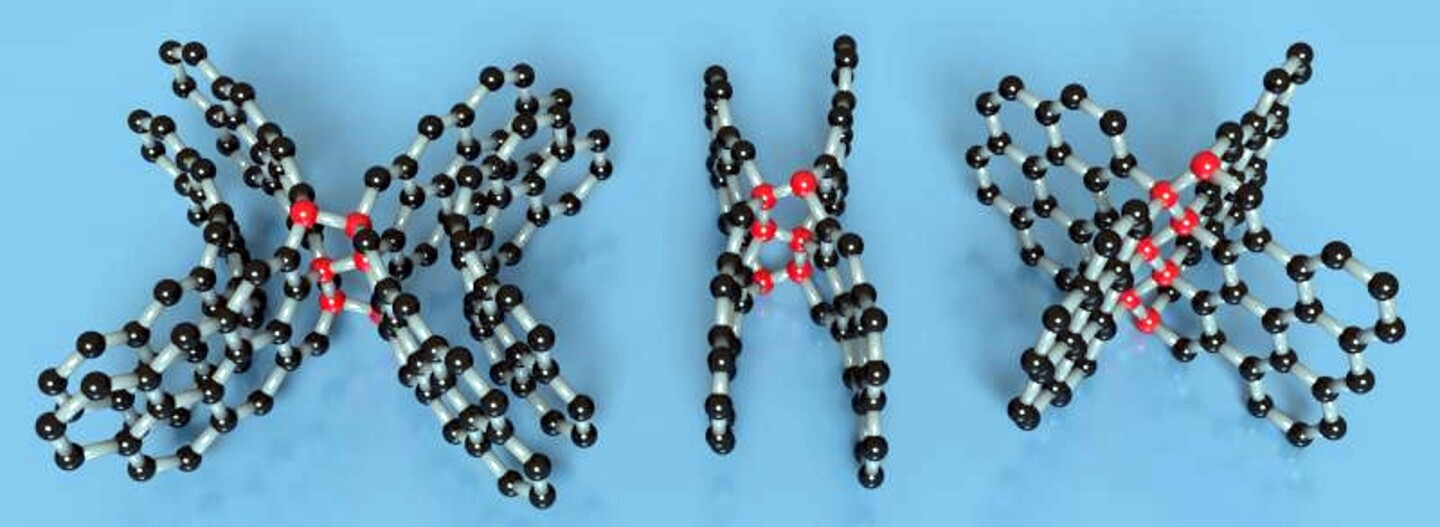
For their experiments, the team subjected the glassy carbon to 250,000 atmospheres of pressure and temperatures of 1,800° F (982° C). Previous attempts to pressurize glassy carbon managed to alter its structure, but it reverted to its original structure on returning to normal pressure. By combining pressure and heat, graphite-like and diamond-like bonds formed as the glassy carbon buckled, merged, and connected into short structures on a nanoscale.
"Light materials with high strength and robust elasticity like this are very desirable for applications where weight savings are of the utmost importance, even more than material cost," says Zhisheng Zhao, a Yanshan University professor. "What's more, we believe that this synthesis method could be honed to create other extraordinary forms of carbon and entirely different classes of materials."
The research was published in Science Advances.
Source: Carnegie Science