Thermoelectric materials work by converting differences in temperature into electric voltage. If two parts of such a material experience significantly different temperatures, electrons within it will flow from the warmer part to the cooler, creating an electrical current in the process. Using these materials, electricity could be generated by the temperature differences on the inside and outside of jackets, within car engines, or even between the human body and the air around it ... just to list a few examples. An international team of scientists have now discovered that an existing material, which behaves like a liquid but isn't one, displays particularly impressive thermoelectric properties.
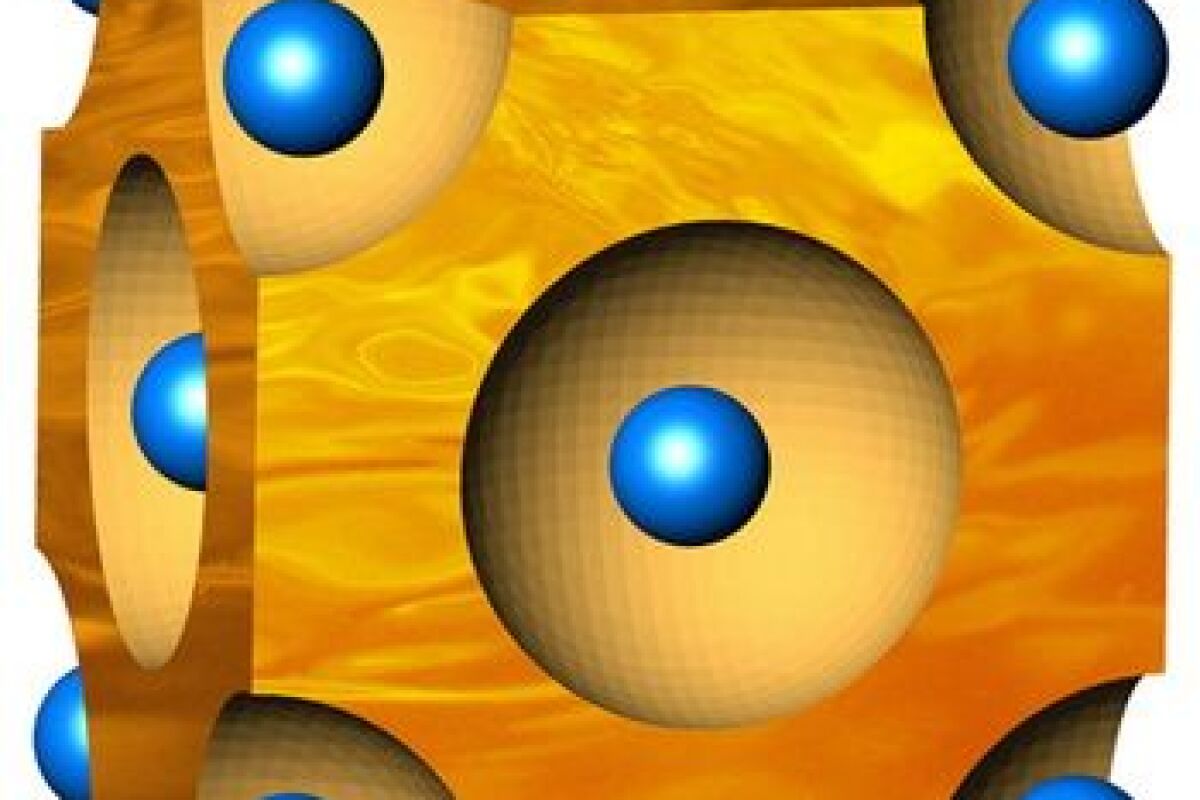
The material is actually a solid, consisting of copper and selenium. The selenium takes the form of a rigid crystalline lattice, which the copper atoms easily flow through – it’s described as being similar in principle to a wet sponge, with the copper playing the part of the water.
Because the thermoelectric effect requires there to be a wide temperature gradient, materials that conduct heat are not well-suited to the task – the more a material is able to disperse heat throughout itself, the sooner that material all reaches one uniform temperature. The material should be a good conductor of electricity, however, as the electrons need to be able to move through it with little resistance.
The copper-selenium material meets both criteria. The free and loosely-flowing copper atoms help drive down its thermal conductivity, while the crystal structure of the selenium boosts its electrical conductivity. According to the scientists, its figure of merit (a rating for thermoelectric efficiency) is one of the highest ever recorded for a bulk material.
As long as approximately 40 years ago, the material was being used by NASA to build spacecraft, although its properties were not fully understood at the time.
The research was led by scientists from the Chinese Academy of Science's Shanghai Institute of Ceramics, working with colleagues from the California Institute of Technology (Caltech), Brookhaven National Laboratory, and the University of Michigan. A paper on their findings was recently published in the journal Nature Materials.
Source: Caltech