A clinical trial of the antibody Aducanumab, conducted by researchers at the University of Zurich (UZH), has yielded positive results, slowing cognitive decline in patients with early-stage Alzheimer's disease. Larger-scale trials of the treatment, which attacks the brain plaques central to the condition, are now underway, across 300 centers in 20 countries.
While our understanding of Alzheimer's is certainly improving, we still don't fully understand what causes the disease. What we do know is that a continued build-up of plaques in the brain occurs some ten to fifteen years before the first symptoms start to manifest themselves.
In an extended research effort, scientists at UZH collected blood from elderly persons, with no cognitive impairment, aged up to one hundred years of age. They then isolated immune cells in the samples, whose antibodies are able to identify the toxic beta-amyloid plaques central to Alzheimer's, but not the amyloid precursor proteins, which are important in the growth of nerve cells.
One such antibody, called Aducanumab, was picked out thanks to its natural occurrence in the body, and its ability to bond with abnormally folded beta-amyloid protein fragments.
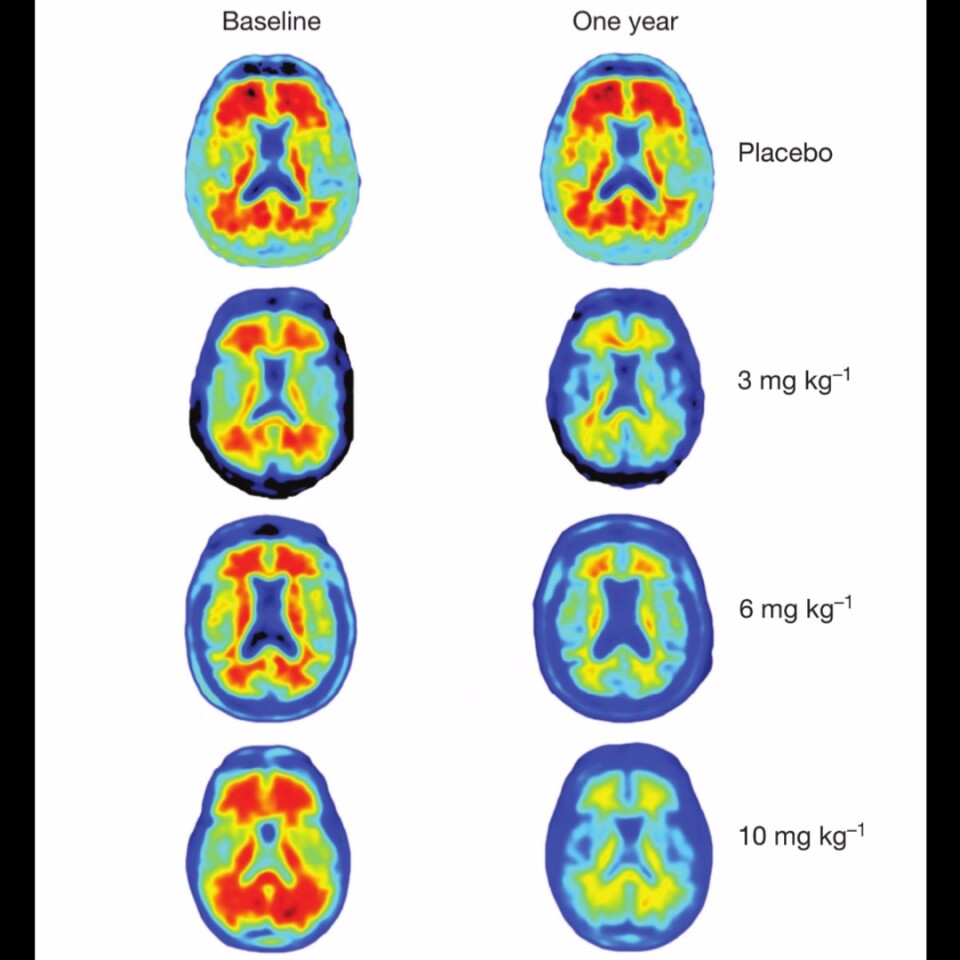
The antibody became the subject of a clinical trial involving 165 patients with early-stage Alzheimer's disease. The participants were split up into groups, and given different amounts of antibody over the period of a year. One set of patients acted as the control group, being given a placebo.
The results were extremely positive, to the extent that the team is tentatively optimistic that the treatment could one day have a big impact on Alzheimer's treatment. While some side effects were observed, including headaches, the effectiveness of the antibody correlated with the dosage administered. After a full year of treatment, almost no beta-amyloid plaques were observed in the patients receiving he highest dosage of the antibody.
The researchers also used questionnaires to investigate how the treatment affected the symptoms of the condition. These looked at the effect of the trial on the cognitive abilities and daily activities carried out by patients.
"Aducanumab also showed positive effects on clinical symptoms," said UZH's Roger M. Nitsch. "While patients in the placebo group exhibited significant cognitive decline, cognitive ability remained distinctly more stable in patients receiving the antibody."
Looking forward, the antibody is now the subject of a much larger study, involving some 2,700 patients. The new trial will further evaluate the treatment's safety and efficacy.
A paper detailing the results of the trial is published online in the journal Nature.
Source: UZH





