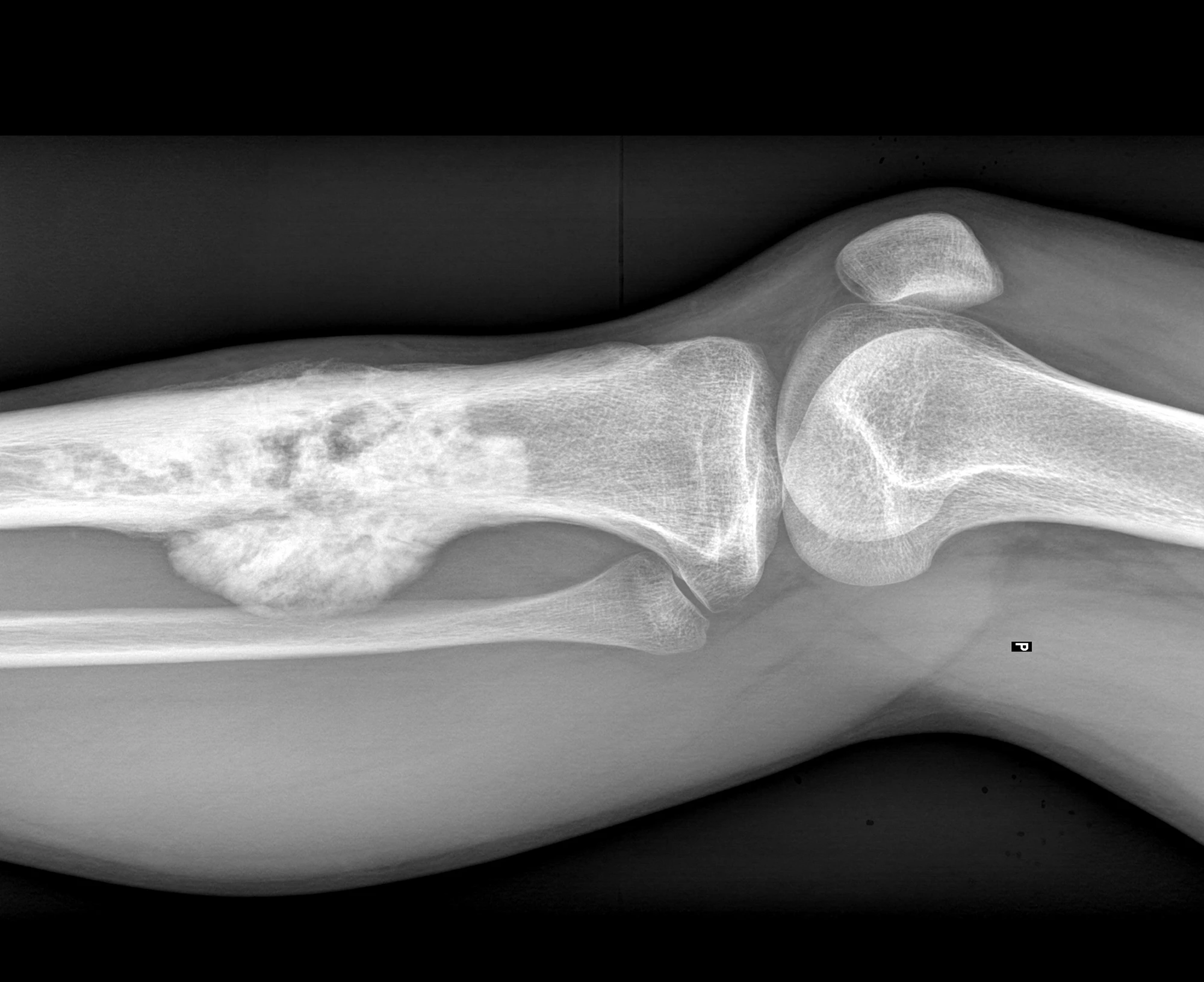
Positive results from clinical trials of an immune therapy in dogs with bone cancer have been used to fast-track the development of the drug to treat children with the same cancer. It highlights how leveraging our genetic similarity can benefit both species.
It’s called comparative oncology, and it’s the study of naturally occurring cancers in companion animals, such as dogs and cats, as models for the treatment of human disease. Spontaneously appearing animal cancers share features with human cancers, like osteosarcoma (bone cancer), prostate and breast cancers, non-Hodgkin lymphoma, and melanoma.
New York-based biopharmaceutical company OS Therapies (OST) is focused on developing immunotherapies for osteosarcoma and other solid tumors. And it’s utilizing comparative oncology to achieve its goal. The company recently announced it had formed a subsidiary, OS Animal Health, to commercialize OST-HER2, its off-the-shelf canine osteosarcoma treatment, using clinical trial data to fast-track a treatment for kids with the same type of cancer.
“Osteosarcoma is the most common canine cancer, affecting more than 40,000 dogs in the US each year,” said Paul Romness, OST’s CEO and President. “Given the recent issuance of a new patent protecting the commercial manufacturing process for OST-HER2 and the rest of our Listeria immunotherapy platform into 2040, we now have a clear commercial opportunity to greatly improve health outcomes in this deadly canine cancer through OS Animal Health.”
HER2 therapies target the human epidermal growth factor receptor 2 (HER2)-expressing cancers, which include breast, esophageal, lung, ovarian, and pancreatic cancers, as well as osteosarcoma. These cancers produce abnormally high levels of the HER2 protein, which accelerates tumor growth and spread. Using modified Listeria monocytogenes bacteria to deliver the DNA therapy to the cells, OST-HER2 stimulates a strong response from the immune system, causing T cells to target the protein.
While osteosarcoma can appear in any bone in a dog’s body, it most commonly appears in the weight-bearing bones of the limbs. It’s aggressive and malignant and often metastasizes (spreads) to the lungs. Tumor resection, including limb amputation, is the usual first-line treatment, while chemotherapy is given in combination with surgery to improve survival time by reducing the risk of metastasis. OS Therapies’ recent clinical trial of OST-HER2 in dogs with limb osteosarcoma, published in Molecular Therapy, showed that the treatment prevented or delayed amputation, slowed tumor growth and metastasis, and improved survival. The results suggest that OST-HER2 is a less invasive and more targeted approach to treating this aggressive cancer.
“It has been my dream since founding the company that OST-HER2 could potentially change the standard of care in osteosarcoma, potentially limiting the need for amputation or surgical resection of the primary tumor,” Romness said in an announcement made after the trial data were released. “With today’s data, we believe we are taking the first steps towards this given that our comparative oncology approach, as a result of the 96% genetic homology between human and canine osteosarcoma, leads us to believe there is significant potential for this canine data to translate into humans in the treatment of frontline and primary metastatic osteosarcoma.”
The drug was featured in a recent PBS documentary, Shelter Me: The Cancer Pioneers, about canine comparative oncology. The official trailer appears below.
As with dogs, osteosarcoma is the most common primary bone cancer in humans. And, like dogs, it can occur in any bone but is more likely to form in the long bones of the legs. In January 2025, OST reported the results of its Phase 2b human clinical trials of OST-HER2 for osteosarcoma that had spread to the lungs and been completely surgically removed. Participants in the trial were aged between 12 and 39. The treatment produced a statistically significant result in 12-month event-free survival (EFS), where an “event” is defined as the recurrence of metastatic osteosarcoma. Additionally, OST-HER2 had a significant and positive effect on overall survival. The study’s findings have not yet been peer-reviewed or published.
“We are extremely pleased with these results of our Phase 2b clinical trial because they show that OST-HER2-treated patients achieved the primary endpoint of 12-month EFS in a statistically significantly higher ratio than comparable historical control, in addition to increasing the likelihood of one-year and two-year survival as compared with comparable historical controls,” said Robert Petit, PhD, the founding scientist behind OST-HER2 and OS Therapies’ Chief Medical and Scientific Officer. “The strong safety profile shown in this study also supports the use of OST-HER2 in this incredibly difficult-to-treat population that has no currently approved therapies.”
As a treatment for osteosarcoma in children, OST-HER2 has received Rare Pediatric Disease Designation (RPDD) from the US Food and Drug Administration (FDA) as well as Fast Track and Orphan Drug designations from the FDA and European Medicines Agency (EMA). This year, OST intends to submit a Biologics License Application (BLA) to the FDA, a formal request for permission to introduce or distribute OST-HER2 as a treatment for osteosarcoma.
“We are laser-focused on getting an Accelerated Approval for OST-HER2 in recurrent, fully resected, lung metastatic human osteosarcoma by year-end 2025 and then using funds obtained from the sale of our pending Priority Review Voucher to expand the potential clinical uses of OST-HER2 throughout the human osteosarcoma treatment paradigm,” Romness said.
Source: OS Therapies