Treating prostate cancer through traditional means such as surgery or radiotherapy carries certain risks, with some patients experiencing impotence, urinary problems and bowel trouble, among other unwanted side effects. Safer and less invasive treatment options could soon be on the table, however, including a novel MRI-guided ultrasound technique that eliminated significant cancers in 80 percent of subjects in a year-long study.
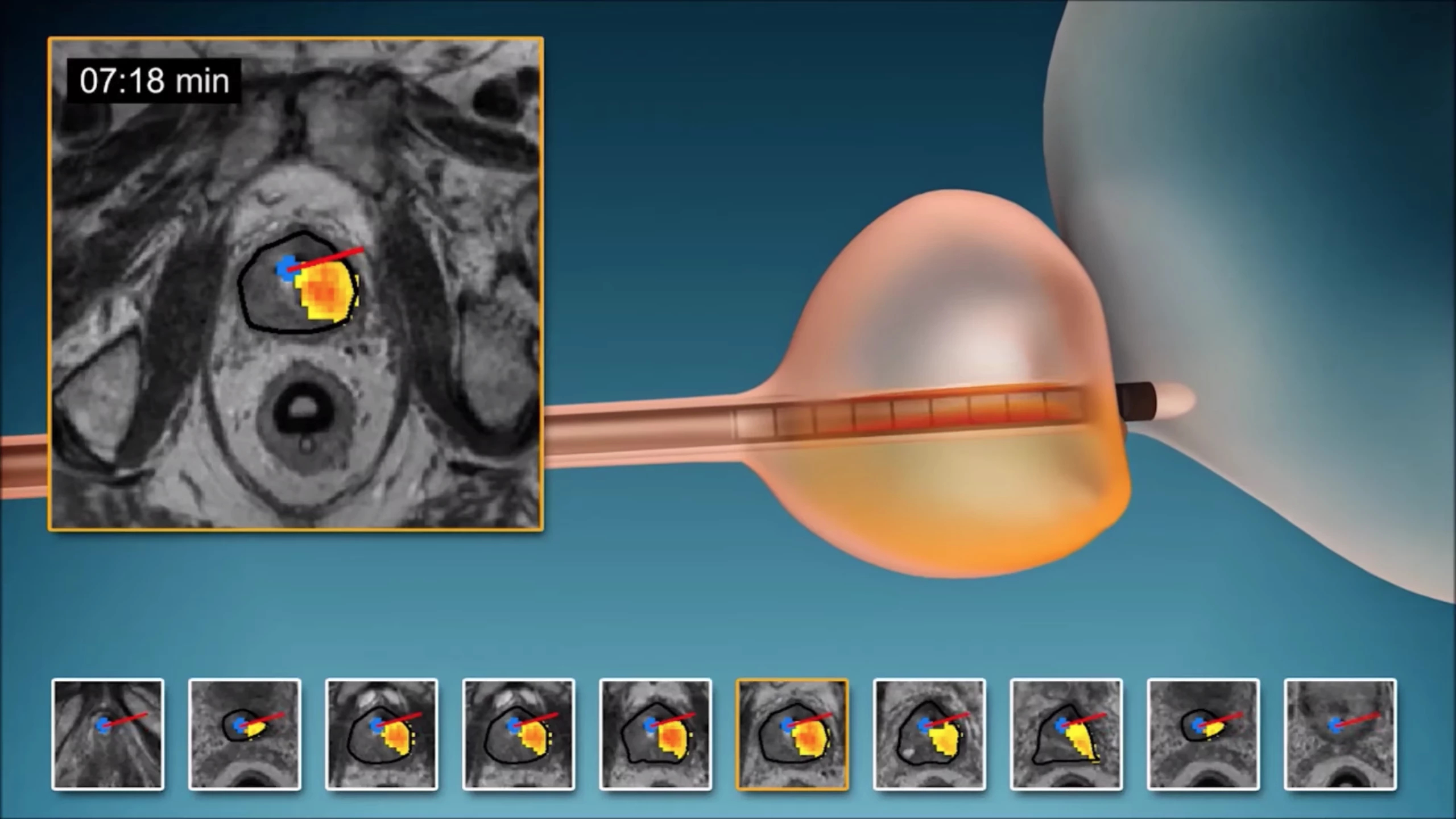
The new technique is called MRI-guided transurethral ultrasound ablation (TULSA) and has been under development for a number of years. The minimally invasive technology involves a rod that enters the prostate gland via the urethra and emits highly controlled sound waves in order to heat and destroy diseased tissue, while leaving healthy tissue unharmed.
These waves come from 10 heating elements built into the length of the rod to treat the entire prostate gland. An algorithm controls which of these elements emit the sound waves at any one time, along with their shape, direction and strength. All of this takes place within an MRI scanner, allowing doctors to keep a close eye on which tissues are being heated and by how much.
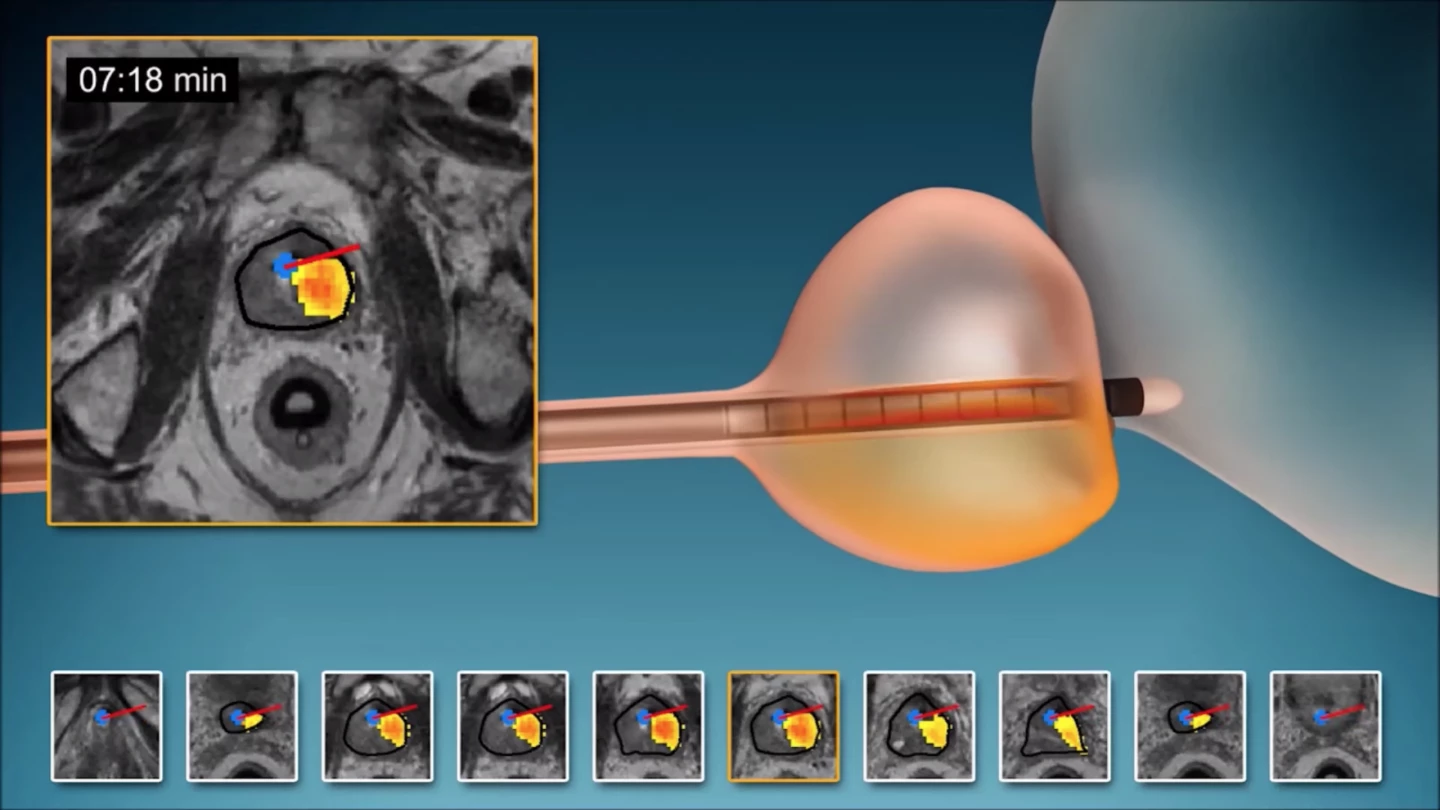
"Unlike with other ultrasound systems on the market, you can monitor the ultrasound ablation process in real time and get immediate MRI feedback of the thermal dose and efficacy," says study co-auhor Steven S. Raman, M.D., professor of radiology and urology at the University of California at Los Angeles. "It's an outpatient procedure with minimal recovery time."
Raman and his team recently put TULSA to the test in a study involving 115 men with low- or intermediate-risk prostate cancer that was confined to the gland. The TULSA treatment was administered to the whole gland for an average time of 51 minutes, with the cancers then reassessed 12 months later.
These follow-up observations revealed some hugely promising results. “Clinically significant cancer” was totally eliminated in 80 percent of the subjects a year after the TULSA treatment. Sixty-five percent of the subjects exhibited no evidence of cancer at all in their biopsies, while prostate-specific antigen (PSA), the key biomarker for prostate cancer, was reduced by a median of 95 percent.
No bowel complications were reported, while the group reported low rates of severe toxicity, low rates of impotence and close to no incontinence (involuntary urine loss from the bladder). Additionally, the technique can be used to treat other benign conditions, such as prostate enlargement.
"There are two very unique things about this system," says Raman. "First, you can control with much more finesse where you're going to treat, preserving continence and sexual function. Second, you can do this for both diffuse and localized prostate cancer and benign diseases, including benign hyperplasia."
The scientists are now working towards further studies to support these exciting results. With TULSA already approved for clinical use in Europe, and having recently received FDA pre-market clearance as safe and effective for prostate treatment in the US, the hope is that it could reach clinical use stateside in the near future.
The scientists presented the research results at this week’s annual meeting of the Radiological Society of North America. An abstract is available online here, while the animation below offers a look at TULSA in action.