Bacteria are fast developing resistance to our best antibiotics, potentially ushering in a new “dark age of medicine” where currently treatable infections become lethal once again. Now, scientists at the National University of Singapore (NUS) have developed self-assembling “nanonets” that can trap and kill bacteria.
The emerging superbug problem is a microcosm of evolution. Essentially, environmental pressures – such as the killing power of antibiotics – leave behind only the bacteria that have a natural resistance to the drugs. As these individuals grow and spread, eventually the entire population ends up with that trait, rendering the drug ineffective. This process has repeated over decades with successive generations of antibiotics, but we’re running out of options.
Along with developing new drugs, scientists are experimenting with alternative methods to control bacteria, ideally ways that they can’t evolve resistance to. That could include lighting, coatings, molecular drills, “poisoned arrows” and liquid metal shredders.
Now, the NUS researchers are adding a new weapon to the arsenal. They’ve developed nanonets that can self-assemble in the presence of certain bacteria, trapping the bugs and making them more vulnerable to antimicrobial molecules.
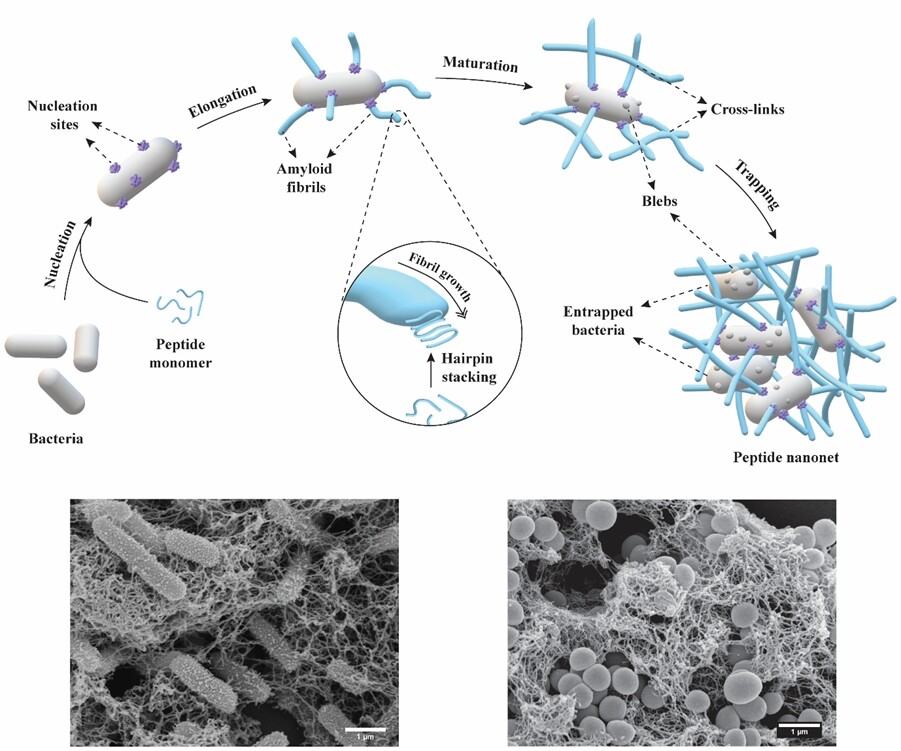
The team designed a series of short peptides, consisting of 15 to 16 residues, which can sit dormant until they detect a specific trigger – two molecules that are key components of the membranes of bacteria. When these molecules appear, the peptide fragments latch onto the bacteria and begin to grow into elongated fibrils, which go on to form cross-links with the fibrils attached to other bacteria. This soon creates a big tangled mess that traps the bacteria. That in turn keeps them from growing and spreading, or can be paired with other antimicrobial molecules to finish them off.
In tests in mice, the nanonets showed significant efficacy against bacteria that are resistant to colistin, one of our last-resort antibiotics. Importantly, they didn’t show any sign of toxicity to the mice.
Not only are these nanonets naturally selective for superbugs, but the peptides comprising them can be tuned to target different bacteria. The team says that this technique shows great promise as a potential new antibiotic treatment, but of course, further work will need to be conducted in the meantime.
The research was published in the journal Advanced Functional Materials.
Source: National University of Singapore





