Researchers have created lab-grown testicle organoids that closely resemble the real thing. The breakthrough provides a promising model for research that may advance our understanding of the organs' development and translate into therapeutic applications for male infertility.
Organoids, lab-grown 3D mini-organs derived predominantly from stem cells, have opened up new ways to model the organs they mimic, including research into disease states and the testing of therapeutic agents. Over the past decade, we’ve seen miniature brains, hearts, lungs, stomachs, and colons that have increased in complexity and functionality. Currently, though, there is no organoid for modeling the testicles.
Researchers at Bar-Ilan University, Israel, have changed that, growing testis (that’s the word for a single testicle) organoids from neonatal mouse cells that generate structures resembling real-life testes.
“Artificial testicles are a promising model for basic research on testicle development and function, which can be translated into therapeutic applications for disorders of sexual development and infertility,” said Nitzan Gonen, the study’s corresponding author.
Dysfunctional testicle development can cause disorders of sex development (DSDs), these days more commonly referred to as intersex, a group of rare conditions involving genes, hormones, and reproductive organs, including the genitals. Dysfunctional development can also contribute to male infertility, a condition where little is known about the genetic and environmental mechanisms underlying it.
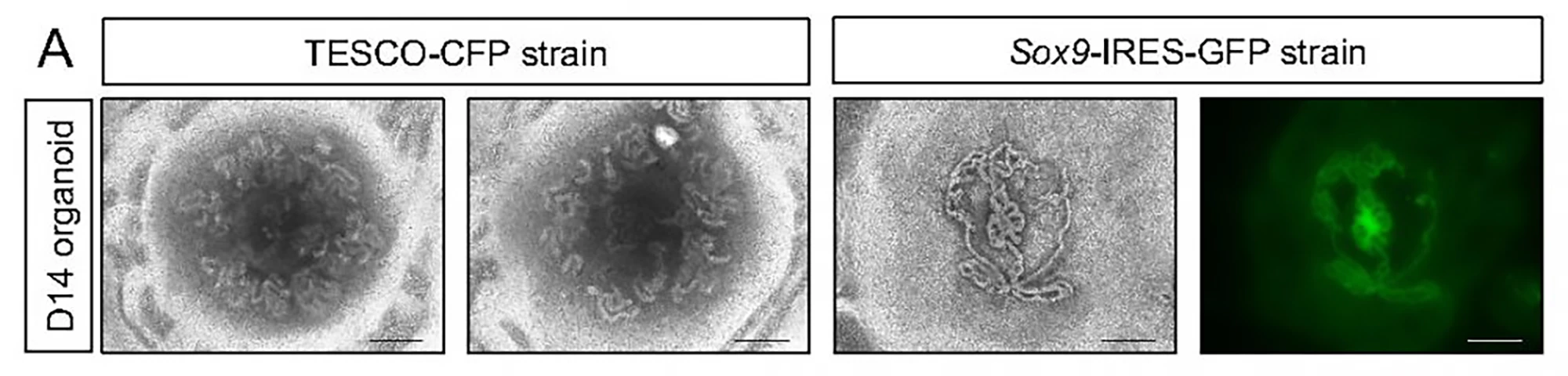
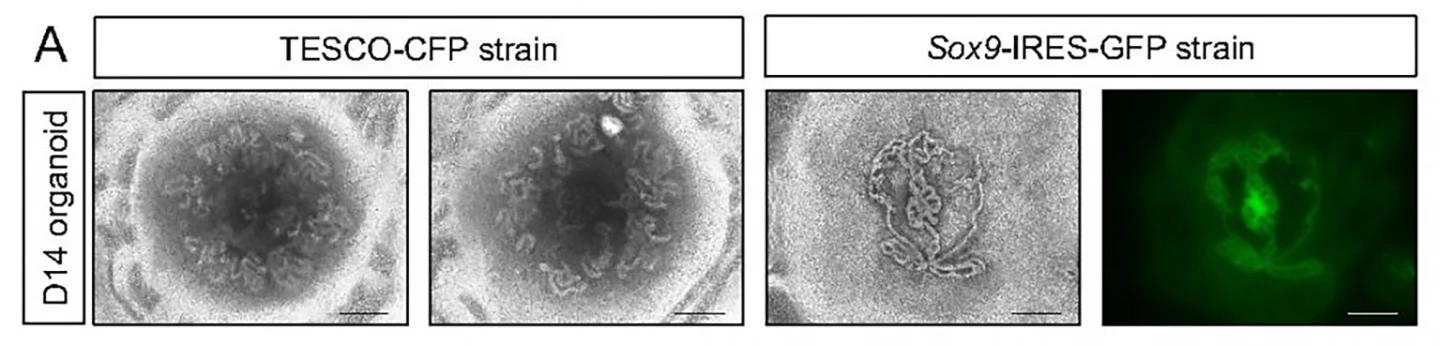
The researchers started with neonatal mouse testes rather than embryonic ones. Compared to neonatal ones, embryonic testes translate to fewer available testicular cells. The mice used in the study had been genetically engineered to allow the researchers to track the presence and state of Sertoli cells, which are essential for testis formation and sperm production and development (spermatogenesis).
Entire testes were harvested from four-to-seven-day-old mice; immature testicular cells were dissociated into single cells and reassembled on a culture medium containing factors normally present in the testes. The researchers used a 3D culture system to support better testicular organoid formation and maintenance. By day two, the cells had formed clear organoids and continued to grow in size for nine weeks, at which point they collapsed.
The testes comprise two main compartments: The testis cords that later become the sperm-producing seminiferous tubules and the interstitial area, mechanical support for the seminiferous tubules, and testosterone producer. Both contain specific cell types. At 21 days, the organoids contained all major testicular cell types, including Sertoli cells, organized in a way that closely resembled real testes. The Sertoli cells formed numerous tubular structures similar to seminiferous tubules.
Despite the relative convenience of creating testicular organoids using neonatal cells harvested from newborn mice, the researchers tried using embryonic cells, which needed to be harvested from pregnant females. Their thinking was this: Neonatal cells have limited use because many disorders relating to testis development and dysfunction occur at the embryonic stage. Using the same technique, they successfully grew testicular organoids from embryonic mouse cells with more well-defined tubular structures than the neonatal-cell-derived organoids. When the researchers tried using adult testicular cells, they couldn’t form an organoid.
While the testicle organoids failed to produce sperm, there were signs suggesting it might be possible. Spermatogenesis is a lengthy process where sperm stem cells undergo meiosis (cell division) to form spermatids that develop into mature sperm. The researchers found low-level expression of meiosis markers in the organoids that appeared to be time-dependent, mostly between days 21 and 42, which may indicate the existence of small quantities of fully mature sperm at the later stages of organoid culture.
The organoids’ close resemblance to actual testes means that they can be used to advance our understanding of the mechanisms involved in sex determination and provide solutions for male infertility.
In future, the researchers plan to produce organoids using human samples. A testicle organoid produced from human cells could, for example, help children being treated for cancer, which can impair their ability to produce functional sperm. They envision harvesting immature sperm cells that are frozen and later used to create a fertile sperm-producing organoid.
The study was published in the International Journal of Biological Sciences.
Source: Bar-Ilan University