The Forest Products Laboratory of the US Forest Service has opened a US$1.7 million pilot plant for the production of cellulose nanocrystals (CNC) from wood by-products materials such as wood chips and sawdust. Prepared properly, CNCs are stronger and stiffer than Kevlar or carbon fibers, so that putting CNC into composite materials results in high strength, low weight products. In addition, the cost of CNCs is less than ten percent of the cost of Kevlar fiber or carbon fiber. These qualities have attracted the interest of the military for use in lightweight armor and ballistic glass (CNCs are transparent), as well as companies in the automotive, aerospace, electronics, consumer products, and medical industries.
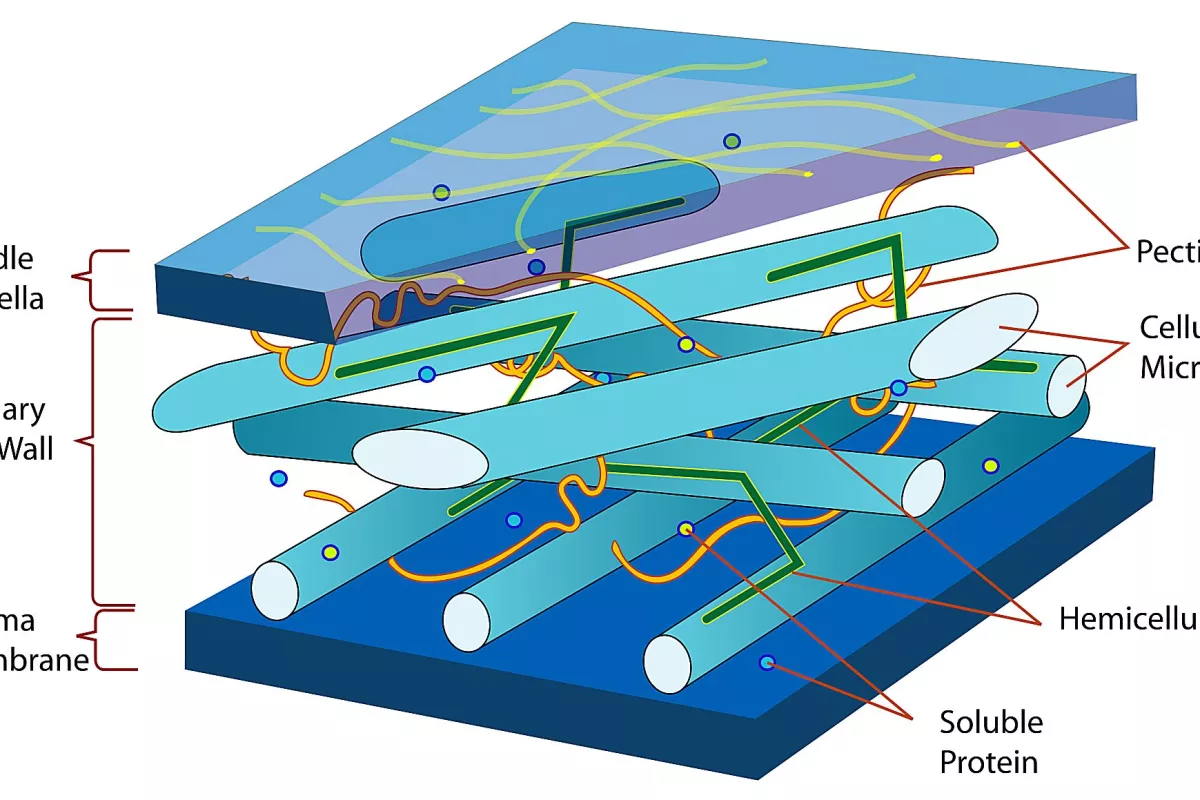
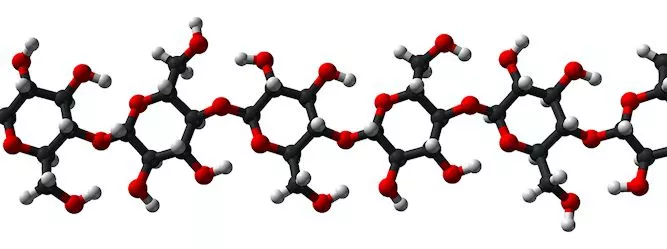
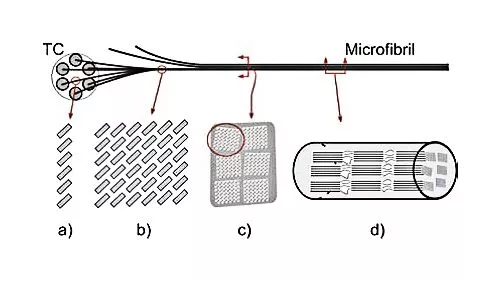
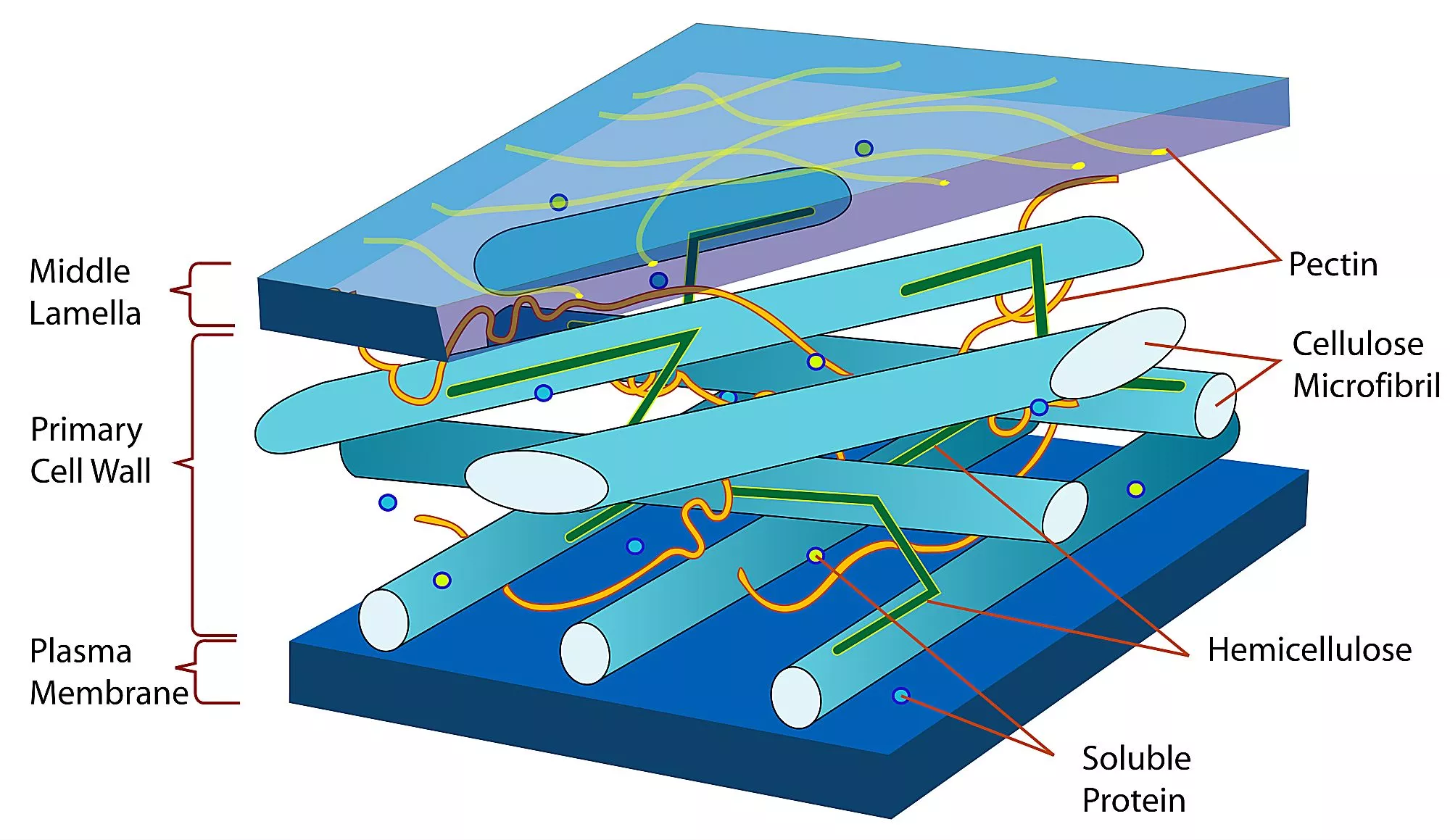
Cellulose is the most abundant biological polymer on the planet and it is found in the cell walls of plant and bacterial cells. Composed of long chains of glucose molecules, cellulose fibers are arranged in an intricate web that provides both structure and support for plant cells. The primary commercial source for cellulose is wood, which is essentially a network of cellulose fibers held together by a matrix of lignin, another natural polymer which is easily degraded and removed.
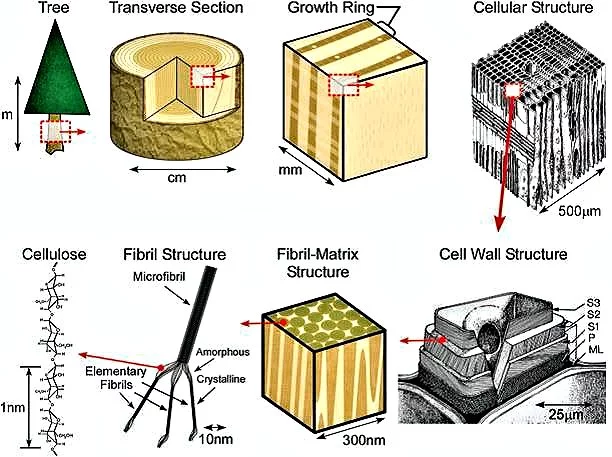
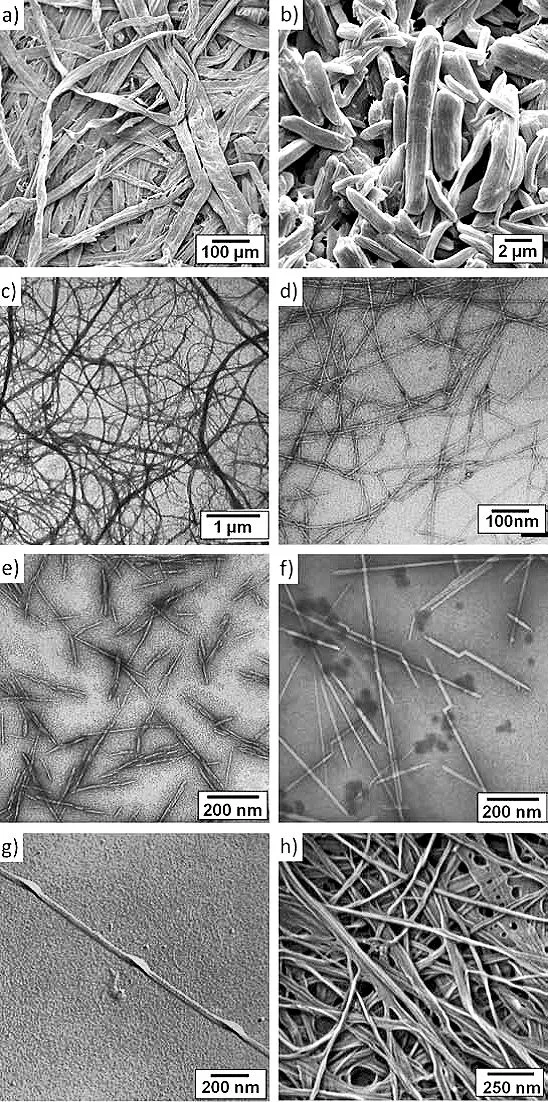
Wood pulp is produced in a variety of processes, all of which break down and wash away the lignin, leaving behind a suspension of cellulose fibers in water. A typical cellulose wood fiber is only tens of microns wide and about a millimeter long.
The cellulose in wood pulp, when dry, has the consistency of fluff or lint - a layer of wood pulp cellulose has mechanical properties reminiscent of a wet paper towel. Not what you might expect to be the source of one of the strongest materials known to Man. After all, paper is made from the cellulose in wood pulp, and doesn't show extraordinary strength or stiffness.
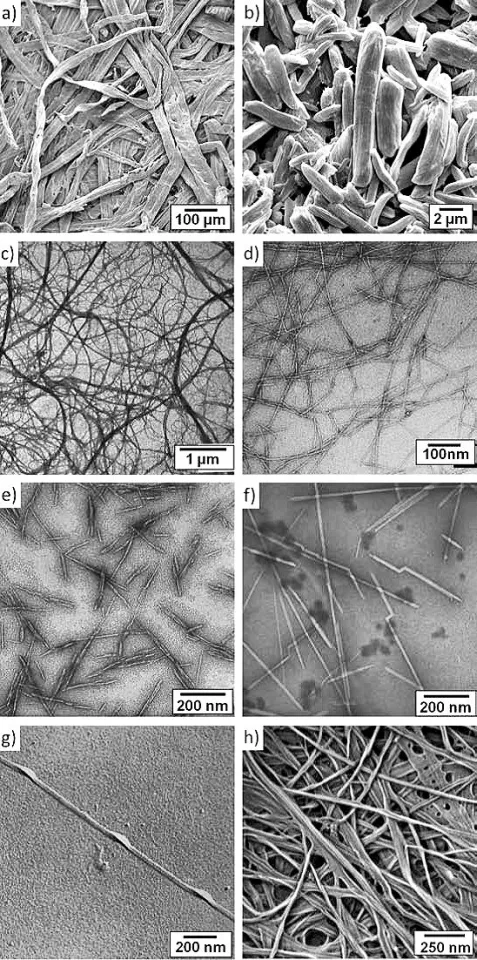
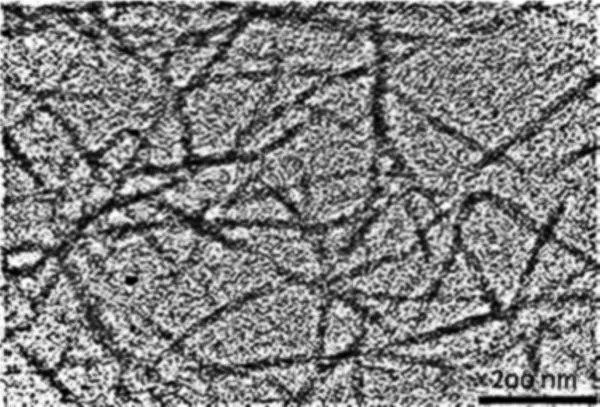
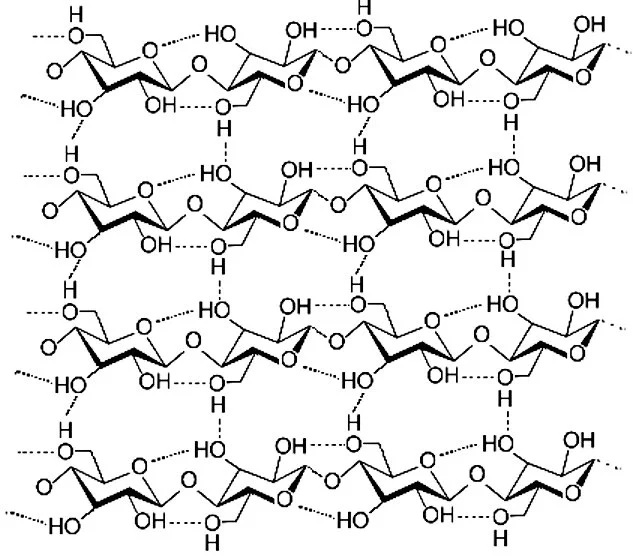
Further processing breaks the cellulose fibers down into nanofibrils, which are about a thousand times smaller than the fibers. In the nanofibrils, cellulose takes the form of three-dimensional stacks of unbranched, long strands of glucose molecules, which are held together by hydrogen bonding. While not being "real" chemical bonds, hydrogen bonds between cellulose molecules are rather strong, adding to the strength and stiffness of cellulose nanocrystals.
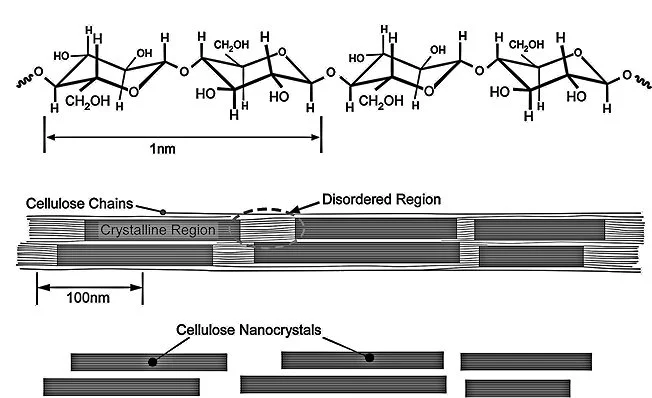
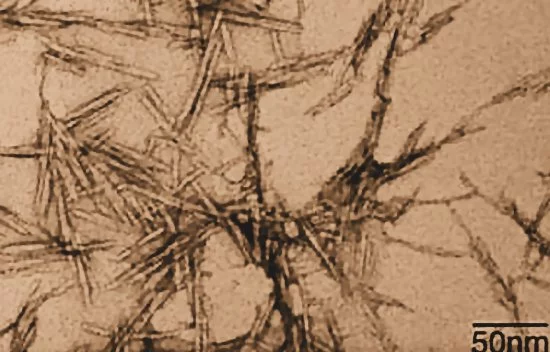
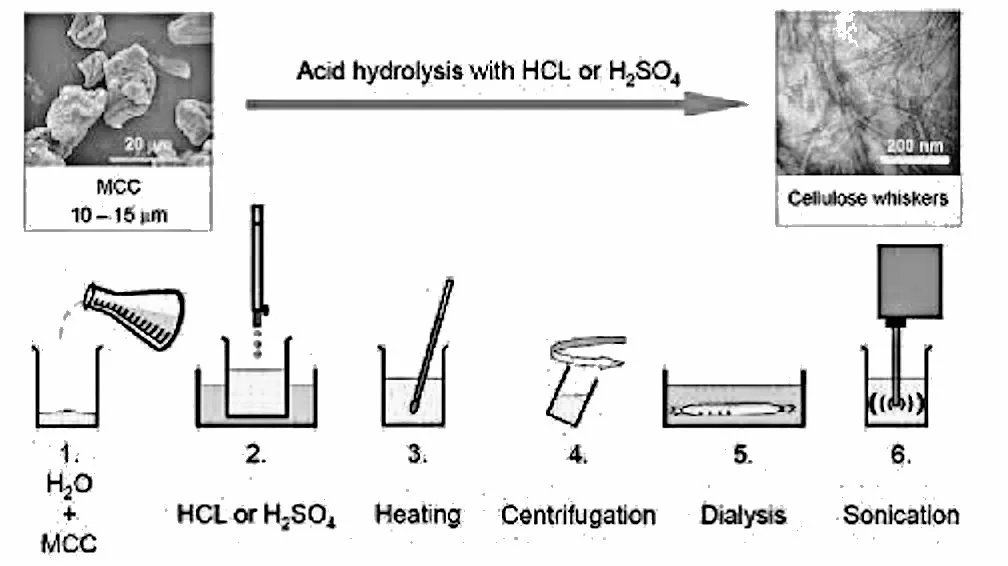
Within these nanofibrils are regions which are very well ordered, in which cellulose chains are closely packed in parallel with one another. Typically, several of these crystalline regions appear along a single nanofibril, and are separated by amorphous regions which do not exhibit a large degree of order. Individual cellulose nanocrystals are then produced by dissolving the amorphous regions using a strong acid.
At present the yield for separating CNCs from wood pulp is about 30 percent. There are prospects for minor improvements, but the limiting factor is the ratio of crystalline to amorphous cellulose in the source material. A near-term goal for the cost of CNCs is $10 per kilogram, but large-scale production should reduce that figure to one or two dollars a kilo.
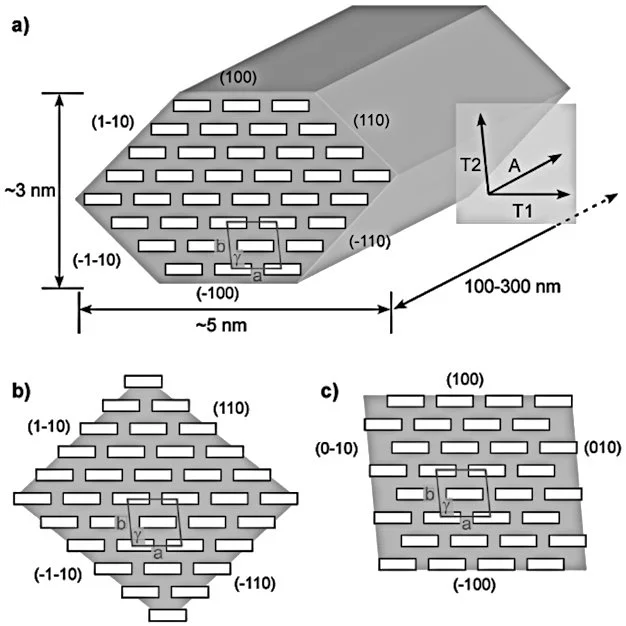
CNCs separated from wood pulp are typically a fraction of a micron long and have a square cross-section a few nanometers on a side. Their bulk density is low at 1.6 g/cc, but they exhibit incredible strength. An elastic modulus of nearly 150 GPa, and a tensile strength of nearly 10 GPa. Here's how its strength to compares to some better-known materials:
- Material...........................Elastic Modulus................Tensile Strength
- CNC......................................150 GPa.............................7.5 GPa
- Kevlar 49..............................125 GPa.............................3.5 GPa
- Carbon fiber.........................150 GPa.............................3.5 GPa
- Carbon nanotubes..............300 GPa............................20 GPa
- Stainless steel.....................200 GPa............................0.5 GPa
- Oak..........................................10 GPa.............................0.1 GPa
The only reinforcing material that is stronger than cellulose nanocrystals is a carbon nanotube, which costs about 100 times as much. Stainless steel is included solely as a comparison to conventional materials. The relatively very low strength and modulus of oak points out how much the structure of a composite material can degrade the mechanical properties of reinforcing materials.
As with most things, cellulose nanocrystals are not a perfect material. Their greatest nemesis is water. Cellulose is not soluble in water, nor does it depolymerize. The ether bonds between the glucose units of the cellulose molecule are not easily broken apart, requiring strong acids to enable cleavage reactions.
The hydrogen bonds between the cellulose molecules are also too strong in aggregate to be broken by encroaching water molecules. Indeed, crystalline cellulose requires treatment by water at 320° C and 250 atmospheres of pressure before enough water intercalates between the cellulose molecules to cause them to become amorphous in structure. The cellulose is still not soluble, just disordered from their near-perfect stacking in the crystalline structure.
But cellulose contains hydroxyl (OH) groups which protrude laterally along the cellulose molecule. These can form hydrogen bonds with water molecules, resulting in cellulose being hydrophilic (a drop of water will tend to spread across the cellulose surface). Given enough water, cellulose will become engorged with water, swelling to nearly double its dry volume.
Swelling introduces a large number of nano-defects in the cellulose structure. Although there is little swelling of a single CNC, water can penetrate into amorphous cellulose with ease, pushing apart the individual cellulose molecules in those regions. In addition, the bonds and interfaces between neighboring CNC will be disrupted, thereby significantly reducing the strength of any material reinforced with CNCs. To make matters worse, water can move easily over the surface/interfaces of the CNCs, thereby allowing water to penetrate far into a composite containing CNCs.
There are several approaches to make CNC composite materials viable choices for real world applications. The simplest, but most limited, is to choose applications in which the composite will not be exposed to water. Another is to alter the surface chemistry of the cellulose so that it becomes hydrophobic, or water-repelling. This is easy enough to do, but will likely substantially degrade the mechanical properties of the altered CNCs. A third approach is to choose a matrix material which is hydrophobic, and preferably that forms a hydrophobic interface with CNCs. While not particularly difficult from a purely chemical viewpoint, there is the practical difficulty that interfaces between hydrophobic and hydrophilic materials are usually severely lacking in strength.
Perhaps the most practical approach will simply be to paint or otherwise coat CNC composite materials in some material that keeps water away. For such a prize - inexpensive strong and rigid materials - we can be sure that innovations will follow to make the theoretical practical.
Source: US Forest Service