Although it's always best to reuse and recycle, many small, simple, low-power electronic devices tend to be single-use. A new paper battery could make them more eco-friendly, as it's activated by water, and it biodegrades once discarded.
Developed by scientists at Switzerland's Empa institute, the battery is intended for use in applications such as "smart" shipping labels, environmental sensors, and disposable medical diagnostic devices.
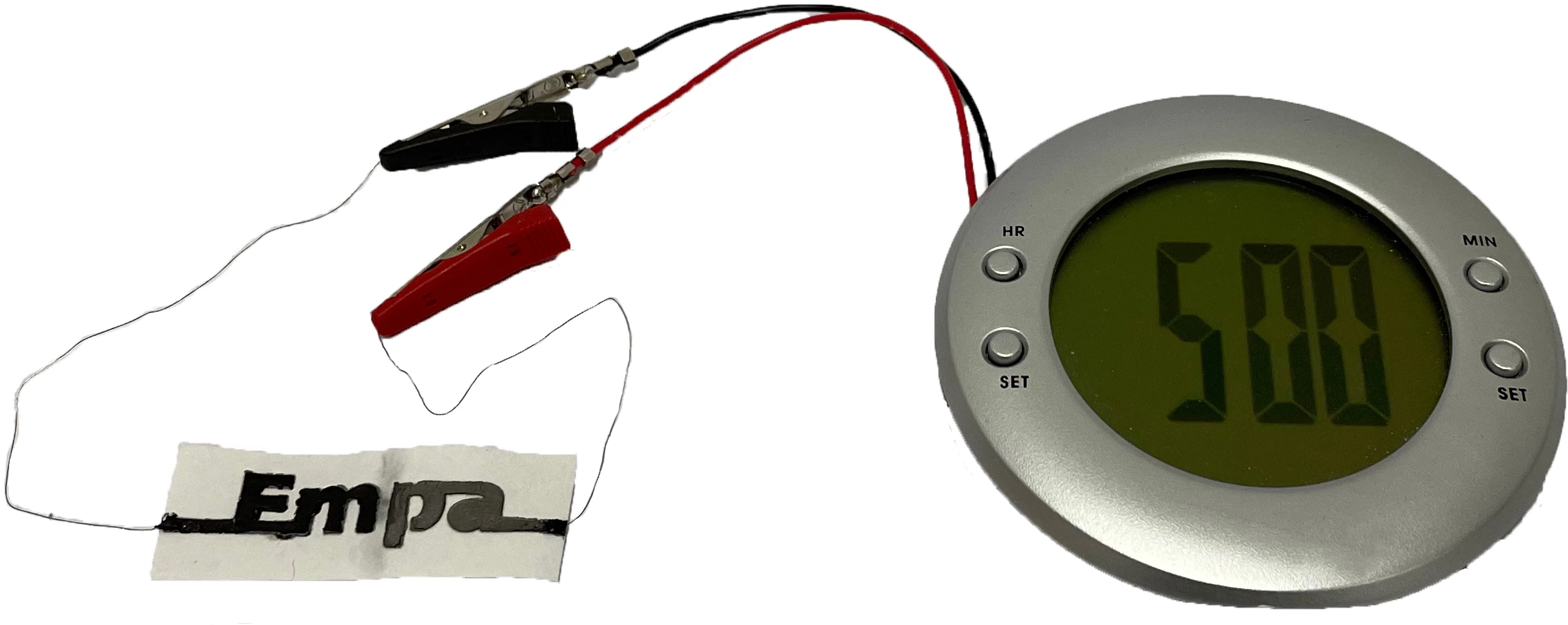
In its current proof-of-concept form, the battery consists of one more linked cells. Each cell measures one centimeter (0.4 in) squared, and its paper substrate is impregnated with sodium chloride (aka table salt). One end of it has a wax coating, to which two wires are attached.
Printed onto one side of the paper is an ink containing graphite flakes, which serves as the cathode. An ink containing zinc powder, which serves as the anode, is printed onto the other side. Covering both inks, on both sides of the paper, is a third ink containing graphite flakes and carbon black – it connects the cathode and anode to the two wires at the one end.
Once a small amount of water has been applied to the battery, the liquid causes the salt in the paper to dissolve, releasing charged ions. As those ions disperse through the wetted paper electrolyte, they cause zinc in the anode to oxidize, releasing electrons.
"These electrons can then be transferred from the zinc-containing anode – via the graphite- and carbon black-containing ink, the wires and the device – to the graphite cathode where they are transferred to – and hence reduce – oxygen from ambient air," Empa explained. "These redox reactions (reduction and oxidation) thus generate an electrical current that can be used to power an external electrical device."
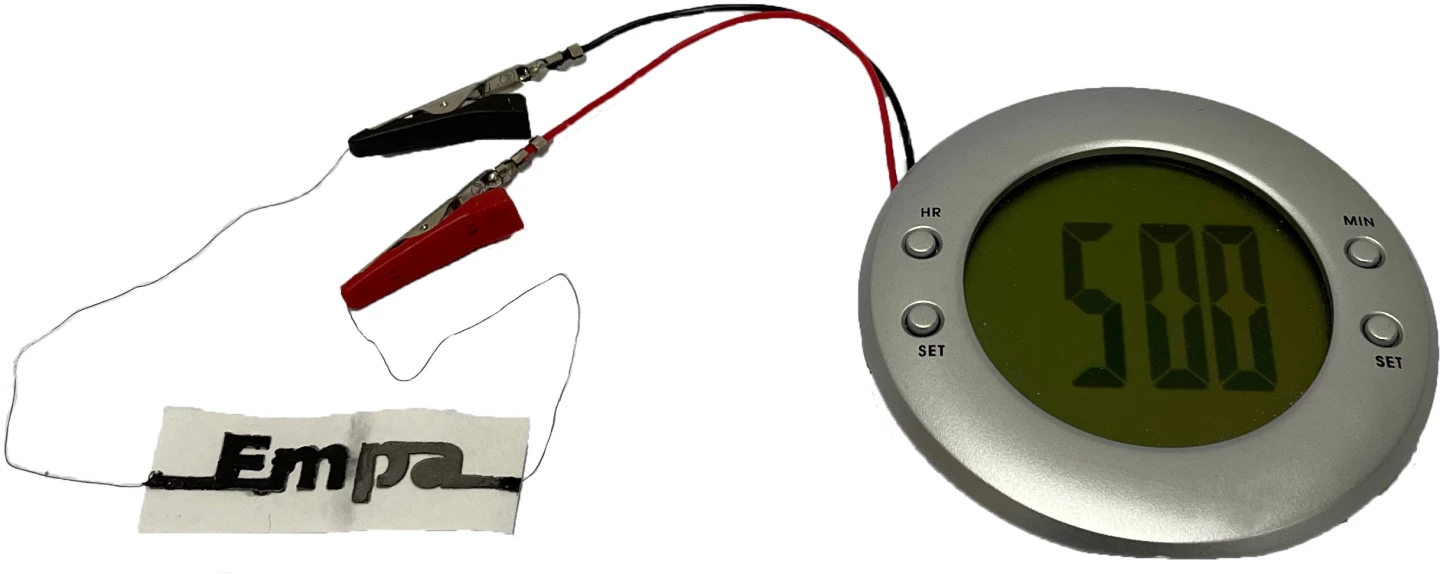
In a lab test, a two-cell version of the battery was successfully able to power a small alarm clock with a liquid crystal display.
Additionally, it was found that just two drops of water were sufficient to activate a single cell within 20 seconds. When not connected to an energy-consuming device, that cell reached a voltage of 1.2 volts. The cell's performance dropped significantly after one hour – as the paper dried out – although it was able to maintain an operating voltage of 0.5 volts for another hour, once another two drops of water were applied.
Lead scientist Prof. Gustav Nyström – who previously developed a biodegradable mini-capacitor – believes that with further engineering, drying of the paper shouldn't be nearly as much of a limiting factor.
The research is described in a paper that was recently published in the journal Scientific Reports.
Source: Empa