A newly discovered receptor switch that boosts bone growth could transform how we treat osteoporosis, by stimulating the body’s own bone-building machinery using a targeted drug and even mechanical force.
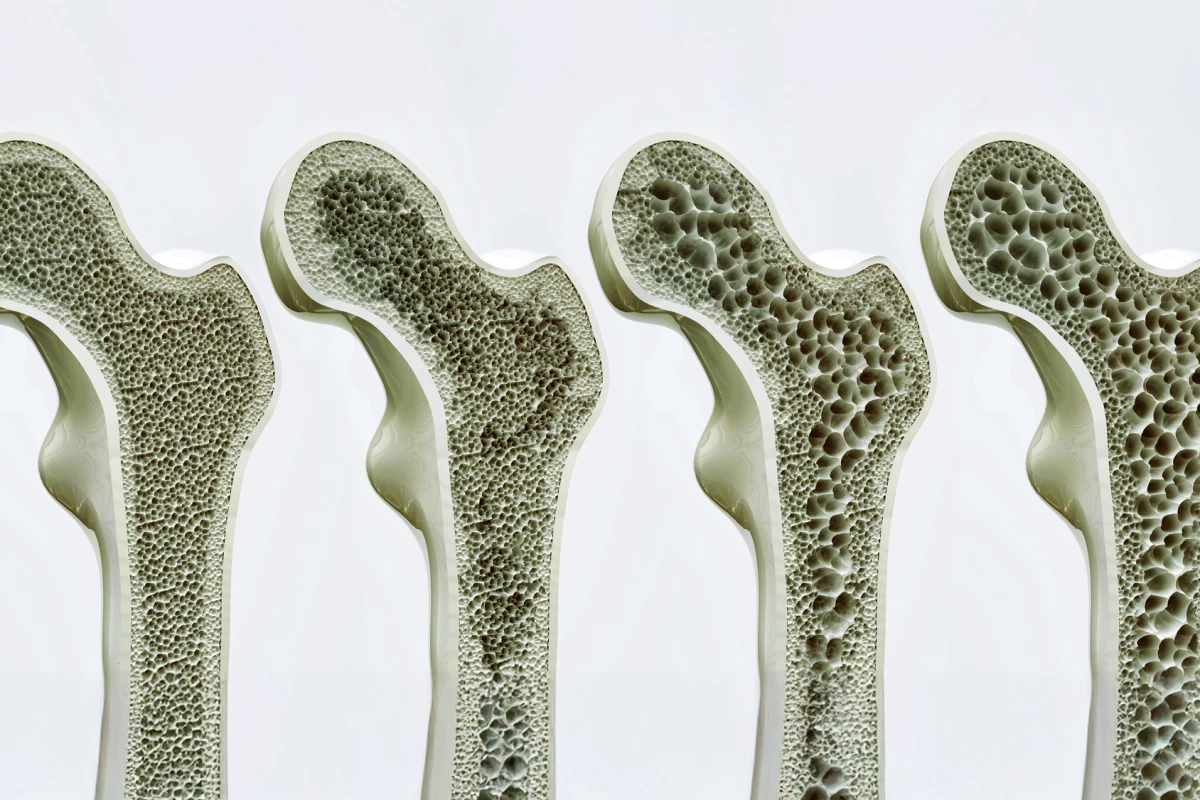
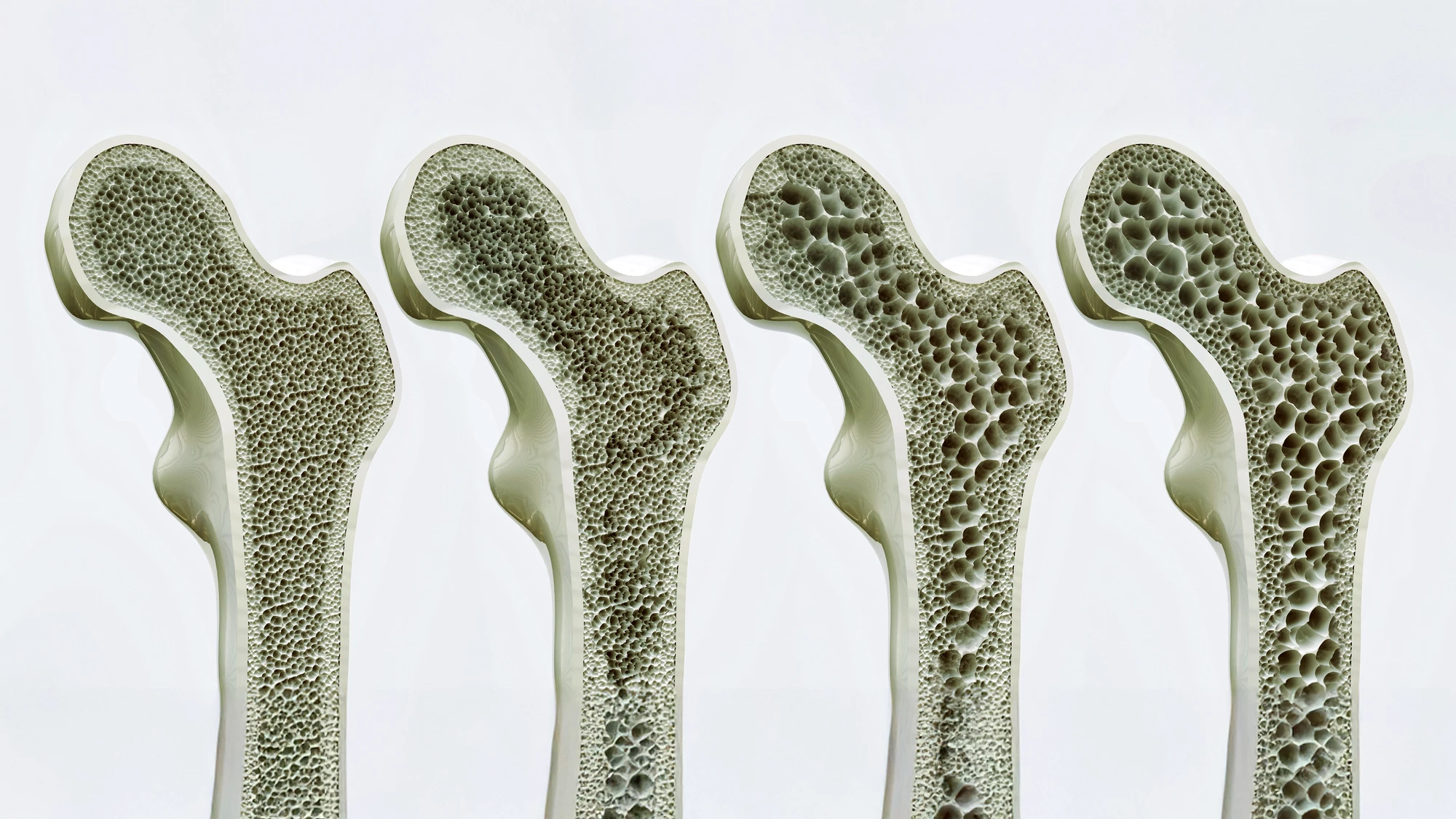
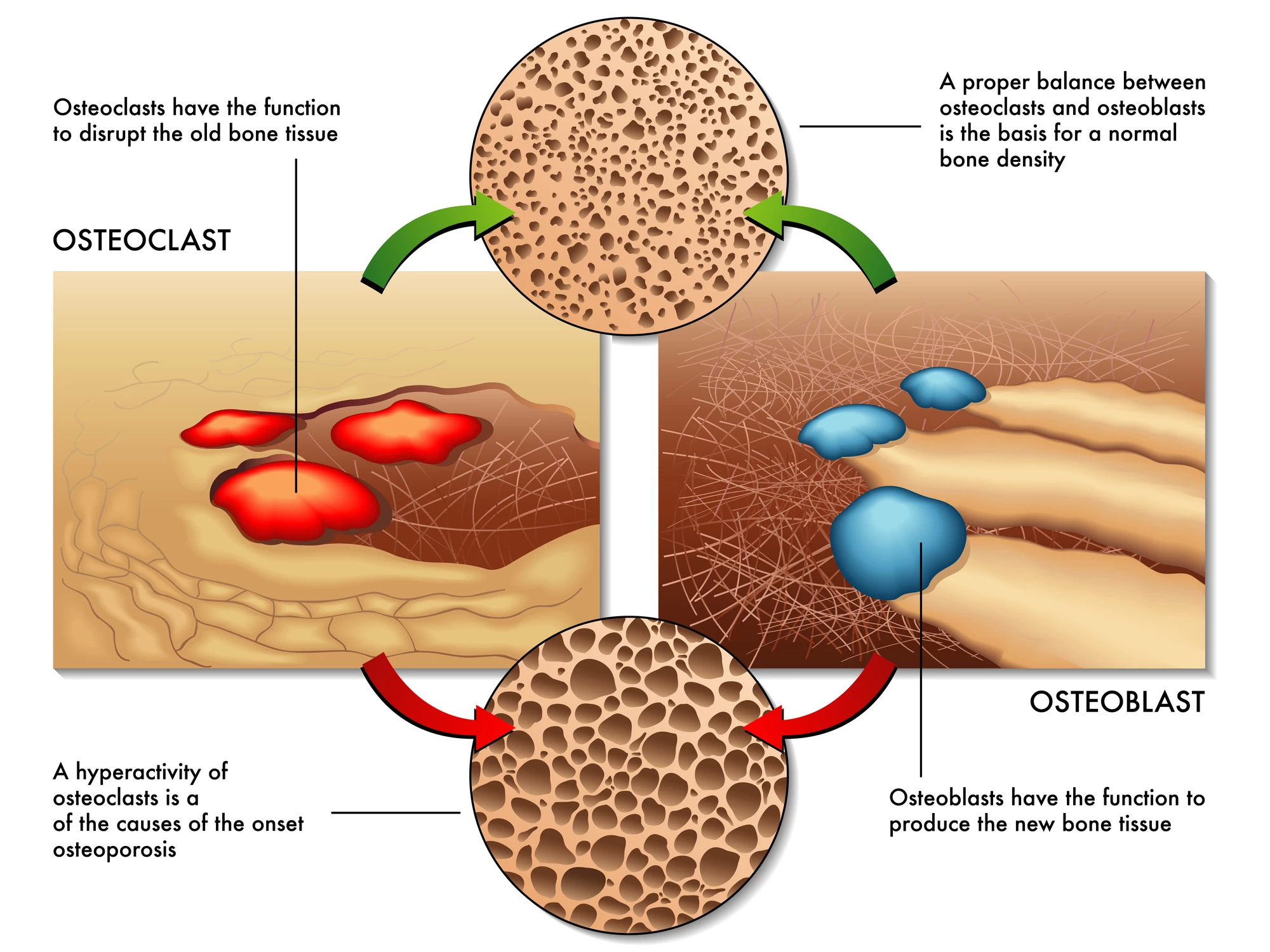
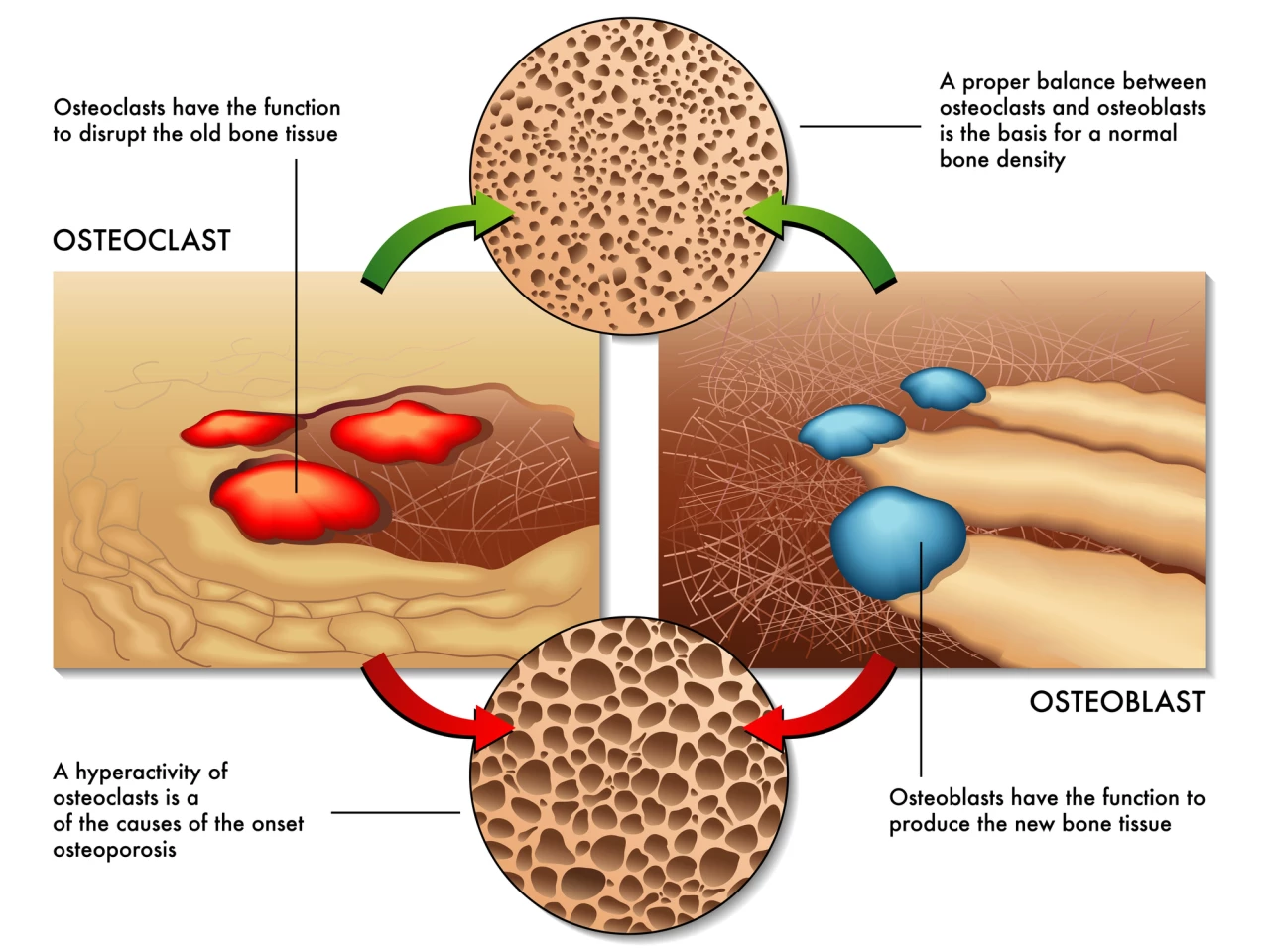
Osteoblasts are bone-forming cells. Osteoclasts, on the other hand, dissolve and break down (resorb) old or damaged bone cells, making room for osteoblasts to create new tissue in areas that are growing or in need of repair. Osteoporosis results from an imbalance between bone formation and bone resorption.
A new study led by the University of Leipzig in Germany has identified a critical regulator of bone formation, the G protein-coupled receptor 133 (GPR133), and a way to stimulate it – opening the door to a new strategy to treat or prevent osteoporosis.
Bone growth is, expectedly, most rapid during childhood and adolescence. Then it slows until our late twenties, when we usually reach peak bone mass. After this, bone density tends to plateau for a time before, generally after age 50, bone breakdown starts to exceed bone formation, gradually decreasing bone density as we age.
Found on cell surfaces, G protein-coupled receptors (GPCRs) can be thought of as molecular switches. When a signaling molecule binds to a GPRC, a G protein is activated, which, in turn, triggers the production of any number of second messenger molecules. The activation of a single G protein can affect the production of hundreds or even thousands of second messengers, affecting various cellular processes and physiological functions.
Previous research identified GPR133 as a potential genetic driver of bone mineral density (BMD) in humans. BMD is the measurement of the amount of minerals, primarily calcium and phosphorus, in a certain volume of bone, and is a key indicator of bone health. Low BMD can increase the risk of fractures, particularly in conditions like osteoporosis. GPR133’s exact role in bone homeostasis isn’t fully known, so the researchers used genetic manipulation, molecular assays, mechanical stimulation, and drug treatment to investigate the molecular mechanisms underlying GPR133’s influence on BMD
Knockout mice were created with the GPR133 gene removed either throughout the body or specifically in precursor cells that differentiate into osteoblasts. These mice developed thinner bones, lower BMD, and increased bone resorption, mimicking osteoporosis. Studies using mouse bone-marrow-derived stem cells and osteoblast precursor cells showed that “switching off” GPR133 meant that osteoblasts didn’t work properly – they couldn’t mature or build bone as well. But when the gene was activated, the cells produced more differentiation markers, proteins that tell us the cell is maturing into a fully functioning osteoblast.
Examining signaling pathways, the researchers found that GPR133 signals through pathways – cyclic adenosine monophosphate (cAMP) and β-catenin – known to be crucial to bone development, remodeling, and maintenance. They also observed that GPR133 activation was mechanosensitive, meaning it responded to simulated physical strain in lab tests, such as stretching or loading. And it interacted with a cell surface protein called PTK7, a “doorbell” that passes messages between neighboring cells. When PTK7 bound to GPR133, it helped activate it; together, PTK7 and mechanical force had a synergistic effect, boosting the signal that helped osteoblasts mature and work properly.
Finally, the researchers tested a small molecule drug, AP503, which selectively activates GPR133. Healthy mice and mice with low BMD (osteopenia) that were given daily injections of AP503 had increased bone density and strength. The injections reversed bone loss in a mouse model that mimics postmenopausal osteoporosis. Combining AP503 with exercise resulted in a synergistic effect, further boosting bone formation.
“If this receptor is impaired by genetic changes, mice show signs of loss of bone density at an early age – similar to osteoporosis in humans,” said the study’s senior author, Professor Ines Liebscher, MD, PhD, from the Leipzig University’s Rudolf Schönheimer Institute of Biochemistry. “Using the substance AP503, which was only recently identified via a computer-assisted screen as a stimulator of GPR133, we were able to significantly increase bone strength in both healthy and osteoporotic mice.”
A prior study led by Leibscher, in collaboration with Shandong University in China, has already found that activating GPR133 using AP503 strengthens skeletal muscle.
“The newly demonstrated parallel strengthening of bone once again highlights the great potential this receptor holds for medical applications in an aging population,” said lead author Dr Juliane Lehmann, also from Leipzig’s Biochemistry Institute.

The use of mouse models only is a limitation of the present study. While informative, mice differ from humans in terms of bone physiology, so translation to clinical settings requires caution. Further, although AP503 was screened for specificity, long-term safety, potential off-target effects, and how the human body affects AP503 after it is administered (pharmacokinetics) remain unknown. And, the effects of GPR133 activation on other tissues or body systems were not extensively explored, which could be relevant for systemic treatment.
However, despite these limitations, the study’s findings highlight some potential real-world applications. GPR133 is a promising therapeutic target whose activation with drugs like AP503 could offer a new class of osteoporosis treatments, potentially with fewer side effects than current options. The findings also open the door to personalized medicine. Some people naturally carry mutations in GPR133, which may predispose them to osteoporosis. Screening for these mutations could identify at-risk individuals for early or preventive treatment.
Beyond treating osteoporosis, GPR133 may have broader applications. Because it’s also involved in muscle development, it could help treat muscle wasting or immobility-induced bone loss, or even bone loss in astronauts exposed to zero gravity.
The study was published in the journal Signal Transduction and Targeted Therapy.
Source: University of Leipzig