Material scientists in China experimenting with carbon in its many forms have conjured up a form of glass so hard that it can scratch the surface of a diamond. As reported by the South China Morning Post, the transparent material is also incredibly strong and has the ability to act as a semi-conductor, opening up some exciting possibilities in the realm of photovoltaics.
Called AM-III, the new material has some parallels with diamonds, both natural and man-made, in that it is formed primarily of carbon atoms. But where diamonds feature an arrangement of atoms and molecules in a perfect lattice structure, AM-III features a more disorganized structure where the atoms and molecules are misaligned, a kind of material known as amorphous.
Types of amorphous materials, or non-crystalline solids, include plastics, gels and, most famously, glass, but the lattermost is not something you'd normally associate with great hardness or strength. The scientists, from Yanshan University in China, sought to bring these characteristics to a glassy material through painstaking trial and error, experimenting with different arrangements of atoms and molecules, and by turning to soccer-ball-shaped molecules of carbon known as fullerenes.
We've seen material scientists study fullerenes to develop ignitable nanoparticles, advanced solar cells and further our knowledge of space, and the authors of the new study found they can also be used as the starting point for the formation of hardy amorphous materials. Fullerenes were subjected to increasing heat and pressure, causing them to be crushed and blended together, with the team carefully dialing up the heat and temperature into uncharted territory until AM-III was formed.
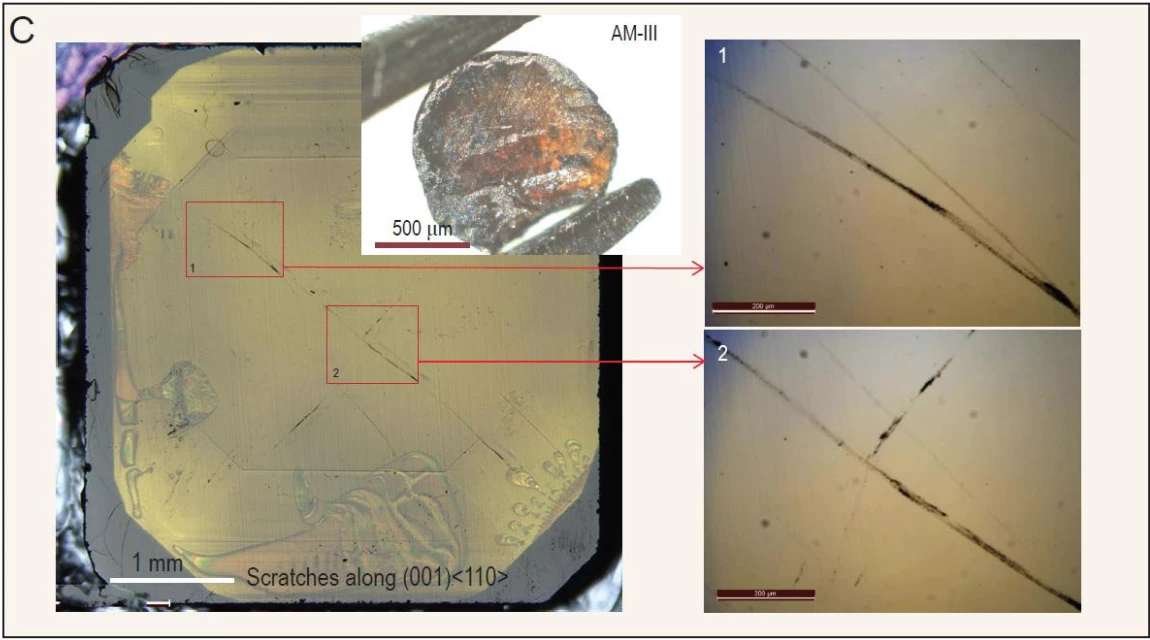
The new material proved to have a hardness of 113 GPa on a Vickers hardness test. For context, mild steel has a Vickers hardness of around 9 GPa, while naturally occurring diamonds rate at around 70 to 100 GPa. The team's comprehensive mechanical testing proved AM-III to be the hardest and strongest amorphous material known so far, and capable of scratching the surface of a diamond.
Further, the material was found to be semiconducting, with a bandgap range of 1.5 to 2.2 eV, similar to commonly used amorphous silicon. This mix of electronic and mechanical attributes makes AM-III an attractive proposition for scientists developing photovoltaic technologies that convert light into electricity, like those seen in solar cells.
The research was published in the journal National Science Review.
Source: South China Morning Post




