A glue-gun-like device that can be used to print biodegradable bone grafts directly into fractures could revolutionize orthopedic surgery, offering personalized implants that speed healing and cut infection risks.
Bone fractures and surgical resection to remove cancer result in larger defects that usually require the use of bone grafts or metal reconstruction to repair. Over the years, science has explored various methods of healing damaged bones, ranging from grafts made from eggshells to piezoelectric scaffolds.
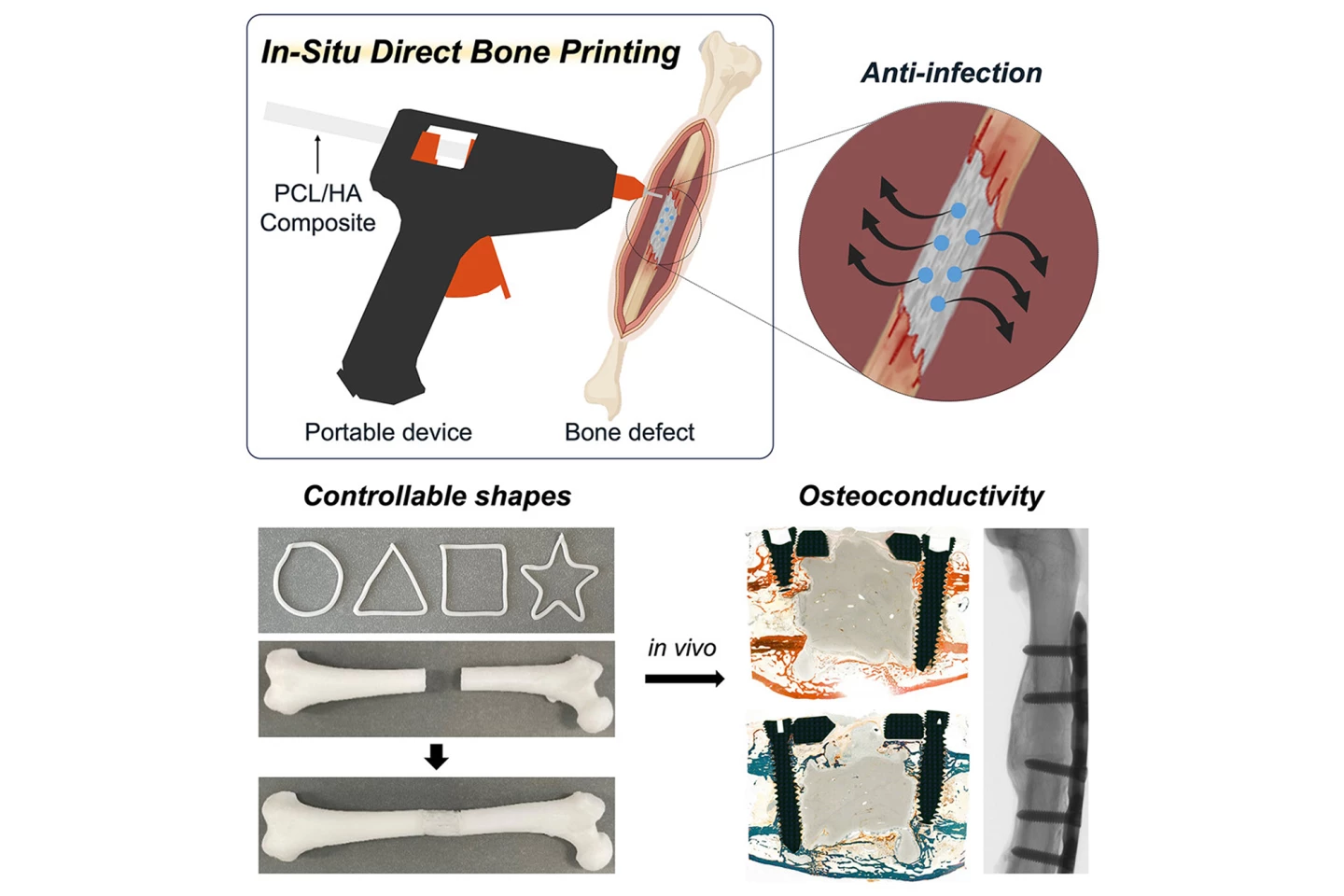
In a new study, Korean and US researchers have developed and tested a modified glue gun that 3D prints bone grafts directly onto fractures and bony defects during surgery.
“Our proposed technology offers a distinct approach by developing an in situ printing system that enables a real-time fabrication and application of a scaffold directly at the surgical site,” said Jung Seung Lee, co-corresponding author of the study and associate professor of biomedical engineering at Sungkyunkwan University (SKKU), South Korea. “This allows for highly accurate anatomical matching even in irregular or complex defects without the need for preoperative preparation such as imaging, modeling, and trimming processes.”
The researchers created a device, modified from a glue gun, capable of low-temperature printing, making it safe for use on living tissue. It uses sticks made from a composite of polycaprolactone (PCL), a biodegradable polymer, and hydroxyapatite (HA), a mineral naturally found in bone, that can be melted down and extruded into bone defects. No toxic solvents were needed. The molecular weight of PCL and the amount of HA were varied to tune strength, elasticity, and degradation rate. They tested compressive strength, bending strength, adhesion to bone, and degradation under simulated body conditions. The researchers also found they could incorporate antibiotics (vancomycin and gentamicin) into the material.
They first tested the bone-healing composite on mouse pre-osteoblasts (which develop into bone-forming cells called osteoblasts) and human bone marrow stem cells to check for toxicity and the composite’s ability to support bone cell growth. Then the researchers moved on to a rabbit model with a femoral bone defect that was too large to heal naturally. They compared their 3D-printed composite with the commercial bone cement that is currently used over a 12-week period.
“Because the device is compact and manually operated, the surgeon can adjust the printing direction, angle, and depth during the procedure in real time,” Lee said. “Also, we demonstrated that this process could be completed in a matter of minutes. This highlights a significant advantage in terms of reducing operative time and improving procedural efficiency under real surgical conditions.”
The researchers found that the composite was stronger and more elastic when HA was added, and HA slowed down the material’s degradation, ensuring the scaffold lasted long enough to support new bone growth. Adhesion to bone improved with higher PCL weight, but decreased slightly when too much HA was added. The antibiotic-coated scaffolds effectively stopped bacterial growth in lab tests, with gentamicin being especially effective. There was no toxicity detected. HA enhanced bone cell attachment, proliferation, and the differentiation of precursor cells into bone cells.
“This localized delivery approach offers meaningful clinical advantages over systemic antibiotic administration by potentially reducing side effects and limiting the development of antibiotic resistance, while still effectively protecting against postoperative infection,” said Lee.
In the animal models, the glue-gun-printed scaffolds supported new bone formation better than traditional bone cement. Micro-CT scans showed that, within 12 weeks after surgery, the printed scaffold produced a stronger, more natural bone structure, with no signs of tissue damage or abnormal inflammation. It is noted, though, that although healing was significantly better compared to bone cement, the defects weren’t completely filled by that time.
“The scaffold was designed not only to integrate biologically with the surrounding bone tissue but also to gradually degrade over time and be replaced by newly formed bone,” Lee said. “The results showed that the printing group exhibited superior outcomes in key structural parameters such as bone surface area, cortical thickness, and polar moment of inertia, suggesting more effective bone healing and integration.”
Cortical thickness in bones refers to the measurement of the outer, dense layer of bone tissue, which is an indicator of bone strength and density. In bone biometrics, the polar moment of inertia is a property of a bone’s cross-section that measures its resistance to twisting (torsional stress).
This proof-of-concept bone grafting method has the potential to personalize orthopedics. Surgeons could carry this personal device into the operating room and print customized bone implants directly where they’re needed, including on irregular defects. Built-in antibiotic delivery could reduce post-surgery infections, which is a major cause of orthopedic implant failures. It would be faster and cheaper, avoiding the costly, time-consuming manufacture of implants. And the device could be adapted with other biodegradable materials or drug additives for different types of bone injuries and patient needs. But there’s a bit more work to do yet.
“Clinical adoption will require standardized manufacturing processes, validated sterilization protocols, and preclinical studies in large animal models to meet regulatory approval standards,” said Lee. “If these steps are successfully achieved, we vision [sic] this approach becoming a practical and immediate solution for bone repair directly in the operating room.”
The study was published in the journal Device.
Source: Cell Press via Scimex






