Researchers have used off-the-shelf components to create a sensor device that is not only cost-effective but can quickly detect 32 different pathogens and has sensitivities on par with the state-of-the-art biosensors used in pathology labs. The novel device could have a range of applications, from monitoring the effectiveness of cancer therapies to predicting the course of viral illnesses.
Diagnosing diseases early benefits patients and doctors. It enables treatment to slow disease progression and reduces the risk of complications, thereby improving long-term health outcomes. With early diagnosis in front of mind, a team from Helmholtz-Zentrum Dresden-Rossendorf (HZDR) research laboratory in Germany has used off-the-shelf components to construct a cost-effective, palm-sized device that can detect 32 different pathogens simultaneously.
To create their novel device, the researchers borrowed from the field of electronics, using field-effect transistors (FETs) as the basic concept. FETs use an electric field to control current flow in a semiconductor. There are three components: source, gate, and drain. Applying a voltage to the gate surface alters its electrical potential and controls the current flow between the source and drain. The device is ‘energized’ only when the gate voltage reaches a certain threshold. Different pathogens generate different electrical potentials and, therefore, different currents. Cancer cells, for example, produce a current different from the flu virus. No significant change in the current means that no disease-relevant biomolecules have bound to the sensor (gate) surface and vice versa.
A major disadvantage of traditional FET-based biosensors is that the test surfaces aren’t reusable, requiring the entire transistor to be discarded after use, which is costly and not very environmentally friendly. To address this issue, the researchers used a separate electrode connected to the transistor’s gate to measure the changes in electrical potential.
“This allows us the opportunity to use the transistor multiple times,” said Larysa Baraban, the study’s corresponding author. “We separate the gate and refer to it as an ‘extended gate’ – that is, an extension of the test system.”
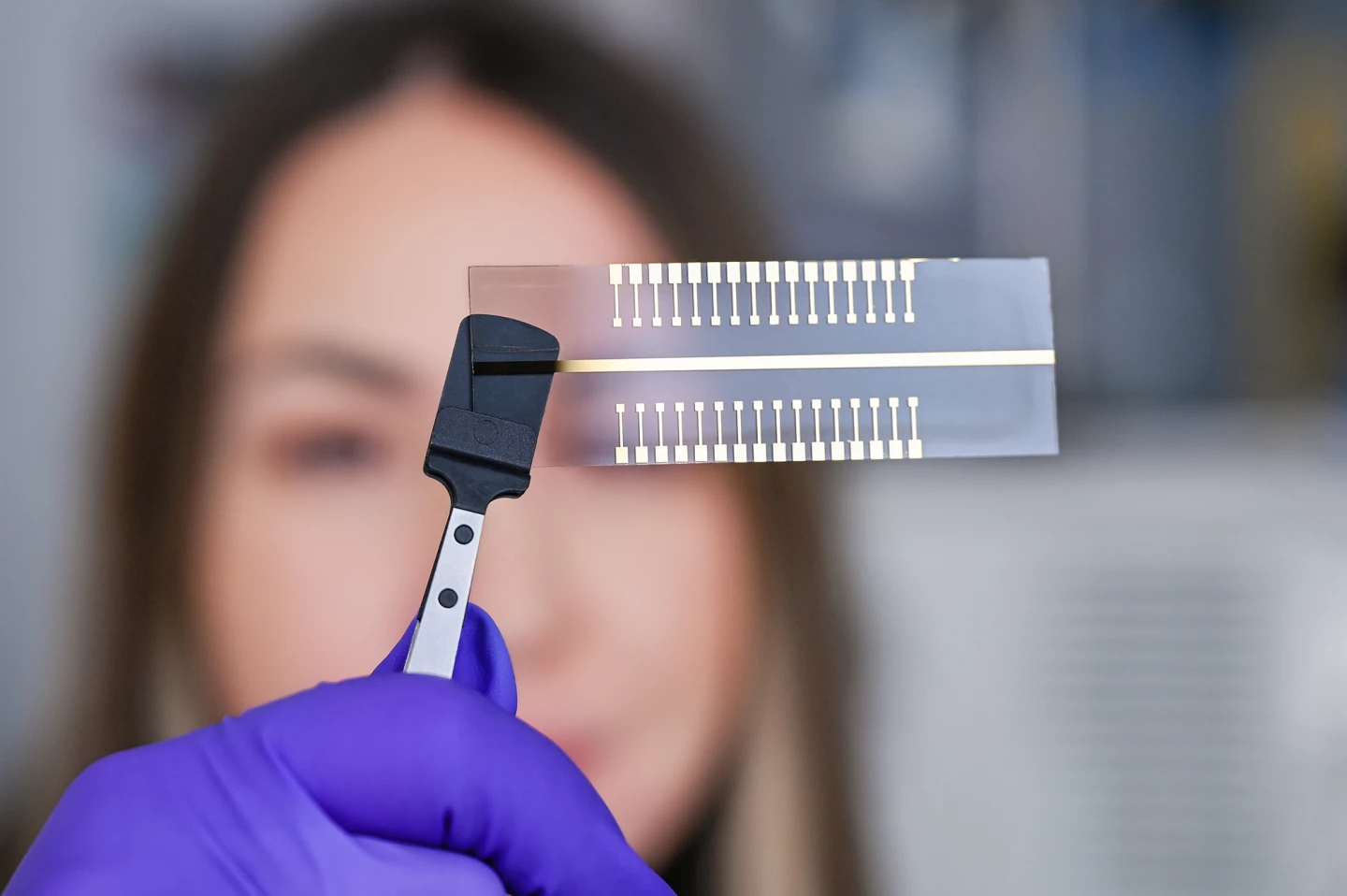
To further improve their system, the researchers created an extended gate with 32 test pads capable of detecting multiple pathogens.
“We, of course, would like this system to carry out several analyses at the same time,” Baraban said. “This means that a sample can be tested simultaneously on each of the pads for a different pathogen.”
The researchers used their device to test for interleukin-6 (IL-6), a protein produced in response to infections and tissue injuries. It’s a useful marker of immune system activation and can be elevated in inflammation, infection, autoimmune disorders, cardiovascular diseases, and some cancers.
“Whether it’s a simple cold or cancer, the concentration of IL-6 changes,” said Baraban. “Different diseases as well as different stages of a disease produce different clinical pictures. That is why IL-6 is very well suited as a marker.”
They found that using a ready-made nanoparticle kit designed for researchers to add gold nanoparticles that concentrated or localized the charge and amplified the voltage signal improved the device’s sensitivity.
“The sensitivity of the tests is considerably higher than when we work without nanoparticles,” Baraban said.
They found that their device produced results quickly and achieved sensitivities and limit of detection (LOD) values comparable to state-of-the-art FET-based biosensors. Indeed, the device had significantly lower LOD – defined as the lowest concentration of an analyte in a sample that can be consistently detected, typically at 95% certainty – compared to the standard enzyme-linked immunosorbent assay (ELISA) method that labs commonly use to detect antibodies in the blood.
The researchers say their cost-effective biosensing device has a range of potential applications, from monitoring the progress of immunotherapies in cancer patients to predicting the severity and course of a viral illness such as the flu or COVID-19.
The study was published in the journal Biosensors and Bioelectronics.
Source: HZDR







