In a discovery that took researchers by surprise, a protein known to play a crucial role in cancer growth has for the first time been observed traveling into cell nuclei to flick switches that make cancers more mobile, aggressive and invasive.
Suppressing this mechanism could potentially help contain a variety of cancers such as lung, kidney, stomach and prostate, making targeted treatment more effective. More than 90% of cancer deaths are the result of the spread of cells to other parts of the body, or metastasis.
Stress is well understood to be a factor in cancer metastasizing. Now, scientists have found that when cancer cells are feeling the pressure, the chaperone protein GRP78 (also often referred to as BIP), which resides in the endoplasmic reticulum (ER), migrates to the nucleus to ‘hijack’ gene activity. This essentially reprograms cell behavior, making cancers more difficult to contain.
“Seeing GRP78 in the nucleus controlling gene expression is a total surprise,” said senior author Amy S. Lee, professor of biochemistry and molecular medicine at the Keck School of Medicine of USC. “When it comes to the basic mechanisms of cancer cells, this is something novel that, to my knowledge, no-one has observed before.”
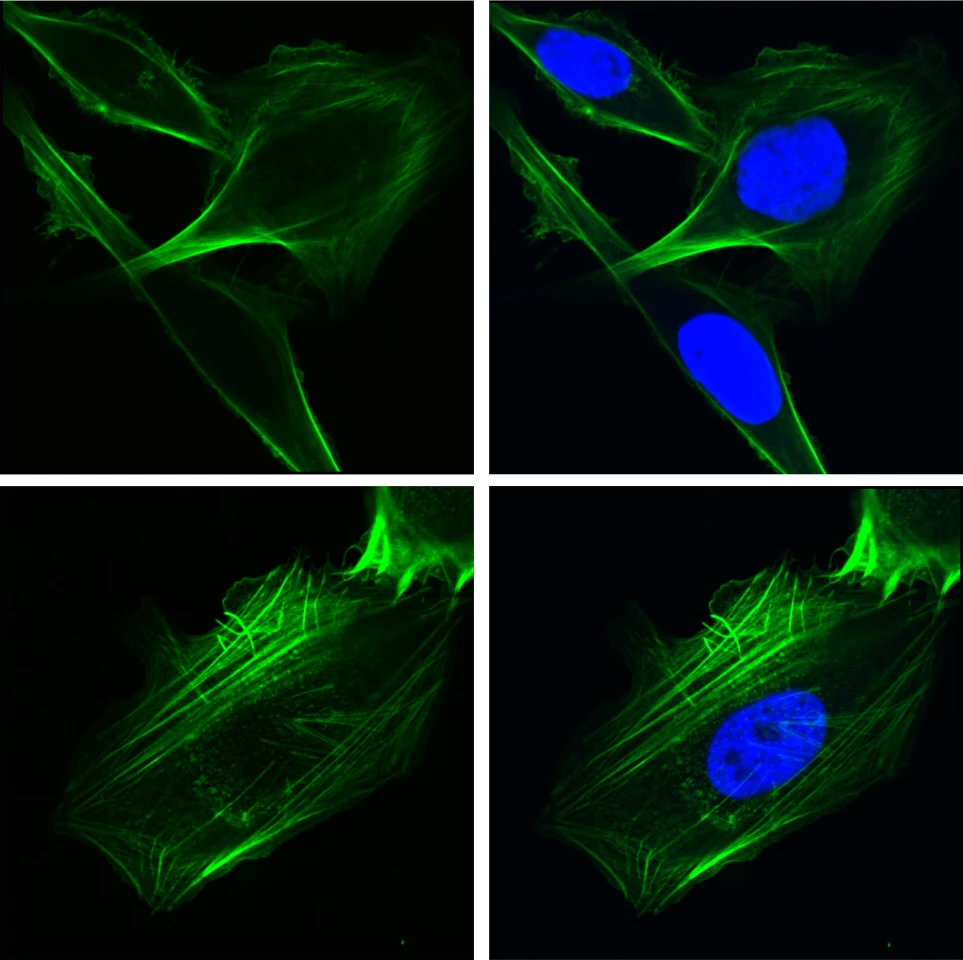
Earlier studies have pinpointed the important role GRP78 plays in cancer cell survival and proliferation, but the ways in which it facilitates this have not been fully understood. The protein has also been implicated in the replication of the COVID-19 virus, increasing the likelihood of mutations that lead to different and more vaccine-resistant strains.
Chaperone proteins aid the folding of other proteins inside the cell, and GRP78 has been thought to only exist in the ER. Through complex RNA sequencing, the scientists were able to see how, under stress, it moved into the ‘brain’ of the cell to regulate the gene EGFR and bind to protein inhibitor ID2.
This ‘hostile takeover’ leads to ID2 being unable to lock down gene expression of EGFR, resulting in an upswing of its activity, which makes cancer cells more mobile and invasive.
“To our big surprise, we found that the key genes being regulated by GRP78 in the nucleus are mainly involved with cell migration and invasion,” Lee said.
Treatments to stop GPR78 in its tracks, or prevent it binding to ID2, presents a new avenue of research and development for scientists. While this study looked at lung cancer cells, GPR78 functions similarly in many other cancers. And there may be other proteins that assume different roles that alter cell behavior when they’re triggered and migrate.
“This is a new concept,” Lee said. “The protein itself is the soldier that does the job, but now we’re thinking it’s not just about the soldier, but also where the solider is deployed.”
The team is now looking at therapies to block the chaperone protein expression, including known ER stress inducer and GRP78 inhibitor YUM70.
The research was published in journal Proceedings of the National Academy of Sciences.
Source: Keck School of Medicine of USC