Researchers have developed a soft conductive electrode that doesn’t require invasive surgery to implant and is resorbed by the body over time. They say their method could be a novel way of treating non-chronic conditions such as cancer and nerve injuries with electrical stimulation.
Therapeutic electrical stimulation to tissues and the nervous system is often used in chronic conditions like Parkinson’s disease or epilepsy, where surgically implanted electrodes provide electrical pulses to specific brain areas. But electrotherapy can also benefit those with non-chronic conditions such as pain, nerve injuries, or cancer.
Researchers from Lund University and the University of Gothenburg have developed a conductive organic electrode that is inserted without invasive surgery, integrates with the body and dissolves over time.
“Our work naturally integrates electronics with biological systems, which opens up possibilities for therapies for non-chronic disease that are difficult to treat,” said Martin Hjort, lead author of the study. “In the study, we used zebrafish, an excellent model for studying organic electrodes in brain structures.”
The researchers created their electrodes using A5, a water-soluble mixed ion-electron polymer with unique qualities: it self-assembles in a gel cast and generates a highly conductive hydrogel that remains stable for several months. It’s also made up of small polymers called oligomers, which gives it better bioresorption properties.
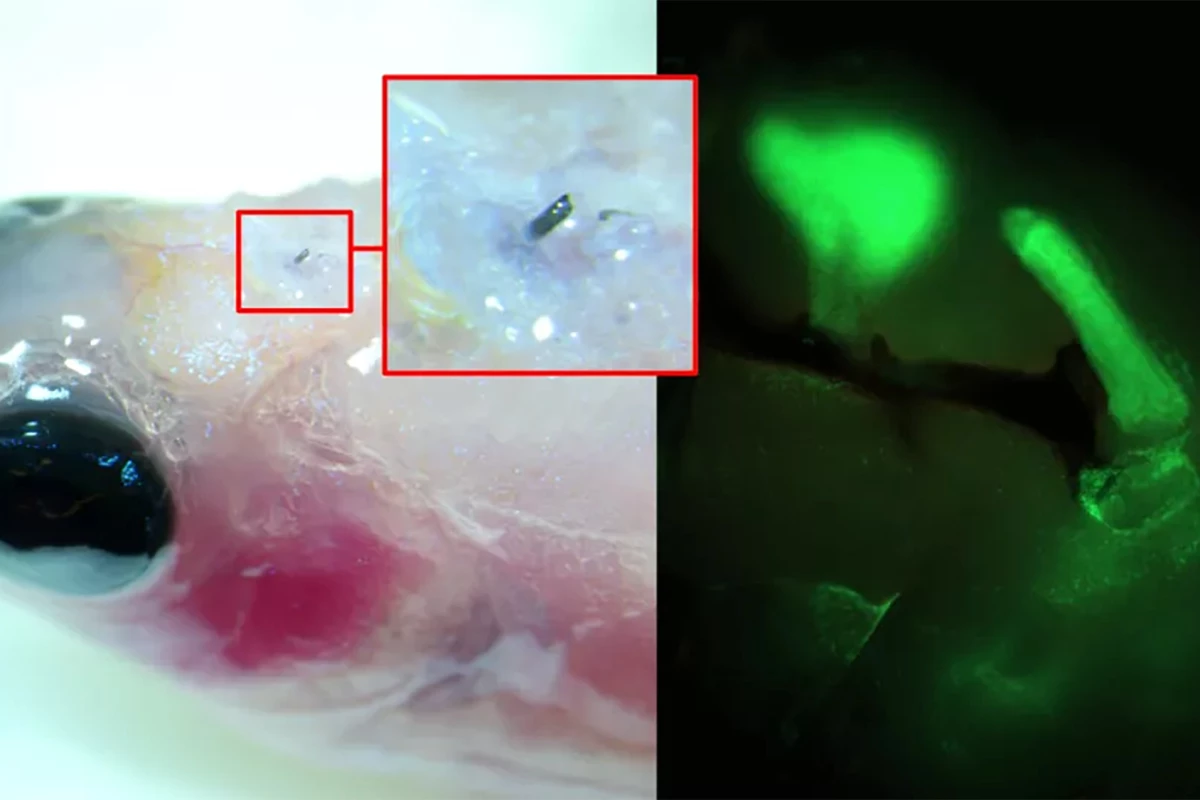
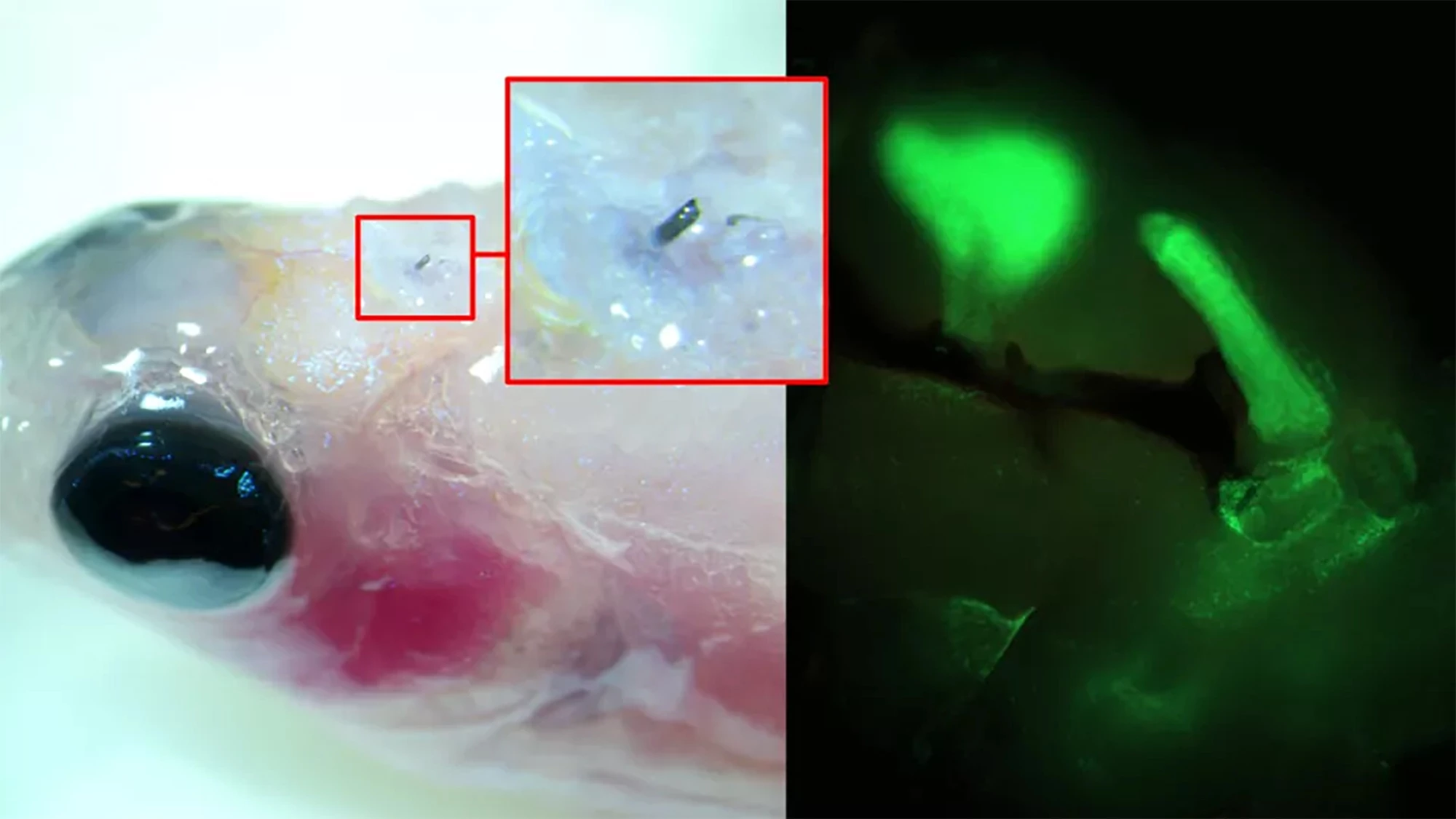
Using a zebrafish, a model of limb regeneration and neuropathy or nerve damage that leads to pain, the researchers injected A5 into the fish’s brain using a syringe with a cannula diameter of 30 µm (A5 nanoparticles are, on average, 80 nm in size). When the A5 interacted with endogenous ions, it arranged itself to form a stable soft electrode.
Over time, the thickness of the soft electrode increased, and dendrites started to grow from it, forming a close connection with the surrounding cells. By applying electrical pulses to brain slices excised from the fish with the implanted electrodes, the researchers could control neural signaling.
“[W]e have developed a technique where a solution of nanoparticles is injected into the tissue using a needle, the size of a human hair,” said Roger Olsson, corresponding author of the study. “These particles, composed of small molecular chains (polymers), then self-organize into a conducting structure and integrate with the body’s cells.”
The conductive structure started to degrade around nine days after insertion before being completely resorbed without causing damage to the fish.
The researchers say their novel minimally invasive electrodes open up possibilities for their use in non-chronic treatments. The next step is to try the procedure on larger – rodent and primate – brains.
The study was published in the journal Nature Communications.
Source: Lund University