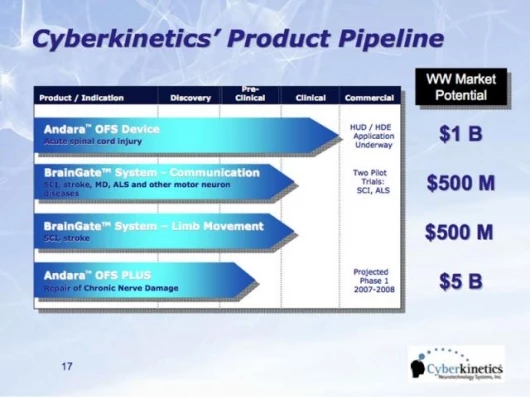
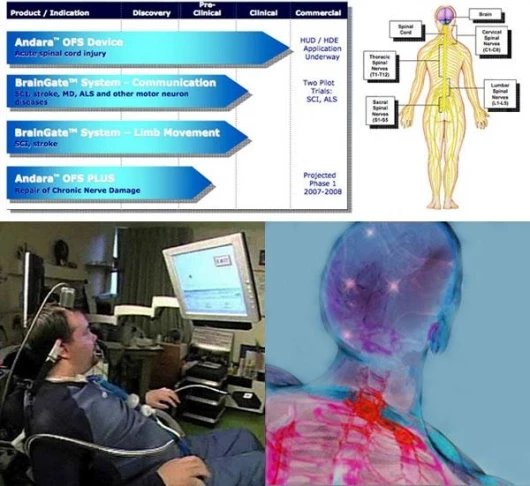
March 9, 2007 We’ve written before about Cyberkinetics Neurotechnology Systems’ BrainGate, a brain-implant device designed to control a computer, assistive devices and eventually, limb movement. The company’s focus is neural stimulation, sensing and processing technology to improve the lives of those with severe paralysis resulting from spinal cord injuries, neurological disorders and other conditions of the nervous system. Cyberkinetics' product development pipeline includes: Andara OFS (Oscillating Field Stimulator) Therapy for acute spinal cord injury, an investigative device designed to stimulate nerve repair and restore sensation and motor function; the; and a pilot program in the detection and prediction of seizures due to Epilepsy. Cyberkinetics has now filed to market its Andara OFS Therapy for Acute Spinal Cord Injury under Humanitarian Device Exemption. Cyberkinetics recently submitted a Humanitarian Device Exemption (HDE) to the Food and Drug Administration (FDA) to obtain market clearance for the implantable Andara OFS System, a nerve growth stimulator. If approved, Andara would be the first commercially available neurotechnology device designed to partially restore sensation and motor function in acute spinal cord injuries by stimulating nerve repair. The company sees it as its first step toward building a Nerve Repair Franchise.
"The Andara HDE-filing is a major milestone achievement for Cyberkinetics because it represents our first therapeutic product and the first step in our strategy to build a valuable nerve repair franchise," said Tim Surgenor, Cyberkinetics' President and Chief Executive Officer. "We are driven by the opportunity to provide this product to people whose lives have been impacted by spinal cord injuries.
"While we await FDA response to our application, we are preparing to launch the Andara OFS Therapy for acute spinal cord injury in an initial group of centers of excellence. If approved, we expect to commercialize the product as early as the fourth quarter of 2007 and generate significant revenues over the next few years. In the coming months, we plan to build out our core sales and marketing team and complete development of the Andara OFS surgeon training materials, reimbursement systems and patient registry program."
The most severe spinal cord injuries result in devastating and permanent loss of sensation and movement, with little prospect for spontaneous improvement. The loss of sensation alone can result in a range of serious health and quality of life problems. Of the estimated 11,000 spinal cord injuries in the United States each year, Cyberkinetics anticipates that fewer than 4,000 of these individuals are likely to be the primary beneficiaries of Andara OFS Therapy. There are currently no approved treatments for spinal cord injury that offer the possibility of returning sensory or motor function. Spinal cord injured patients generally face poor prognosis -- several surgical procedures and extensive rehabilitation programs, which are extraordinarily expensive. Into this context, we expect that our Andara OFS Therapy will make a significant difference in the course of these patients' lives.
About the Andara OFS Technology Platform
Cyberkinetics' Andara OFS Therapy is based on initial research by the Center for Paralysis Research at Purdue University and is intended to improve or restore tactile sensation and some movement in those with quadriplegia and tetraplegia due to recent spinal cord injuries by promoting nerve fiber repair. The OFS device must be implanted within 18 days of a severe spinal cord injury. AndaraOFS Therapy has been shown in published randomized controlled preclinical studies to restore sensation and some motor function in a large animal model. Results of a ten-patient clinical study were published in the Journal of Neurosurgery: Spine in January of 2005 and reported statistically significant improvements in assessments of ability to move and feel at 12 months after treatment compared to baseline.
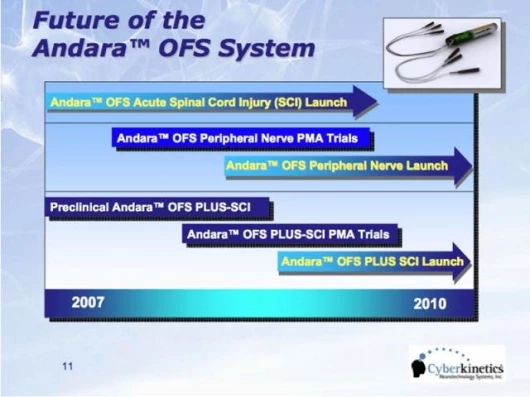
Cyberkinetics expects to expand the use of the OFS Therapy to include the treatment of peripheral nerve injuries, strokes and traumatic brain injuries. The Company's goal is to develop Andara OFS Therapy into a platform applicable to a wide range of nervous system injuries. For example, the AndaraOFS device is currently being tested in preclinical studies in combination with a nerve growth factor. This Andara OFS device-drug combination product may one day be used to treat nerve injuries that are months or years old
Additional information about the Humanitarian Device Exemption is available here.
For those interested in hearing about the very latest on Andara, Surgenor will present at Cowen and Company’s 27th Annual Health Care Conference at 11:00 a.m. (ET) on Thursday, March 15, 2007, at the Boston Marriott Copley Place in Boston, Massachusetts.
In his presentation, Mr. Surgenor will provide an update on Cyberkinetics’ progress with the Andara OFS (Oscillating Field Stimulator) Therapy. To access a live audio webcast at the date and time of the Company’s presentation or a replay of Mr. Surgenor’s presentation go here.