In 2012, a two atom thick layer of silica glass was accidently made in the laboratory of Cornell professor David Muller, allowing the atoms in a glass to be seen individually for the first time. Now, Guinness World Records has identified this ultimately thin glass as a 2014 World Record.
While best known for informing the world that the world's smallest dog is 3.8 inches (9.7 cm) high, or that the longest chocolate sausage ever made was 250 feet (76.2 m) in length, Guinness World Records (better known in the US as the Guinness Book of World Records) also casts its eye on remarkable facts and achievements in science and technology. In selecting new records for its 2014 book, Guinness has focused in on the thinnest piece of glass, the result of a processing accident in a Cornell laboratory engaged in studying the properties of graphene. The Cornell glass is two atoms in thickness, and shows the lack of long-range order expected of a two-dimensional glass.
The silica glass (silicon dioxide, or sand) layers discovered by Prof. Muller's team were grown by accident while growing graphene on a copper film mounted on a quartz (crystalline silica) substrate. The graphene layer was being grown using chemical vapor deposition, a method in which a gaseous hydrocarbon decomposes at a highly heated growth surface, thereby providing a source of carbon with which to grow the graphene layer.
When the researchers inspected the graphene layer, they noticed that a portion of it was discolored. On testing, the discoloration turned out to be a one-molecule thick layer of silica glass. While reproducing the conditions for growth of the glass has not yet been successful, Prof. Muller speculates that the cause of the unusual growth may have been a tiny air leak into the chamber just prior to beginning to grow the graphene.
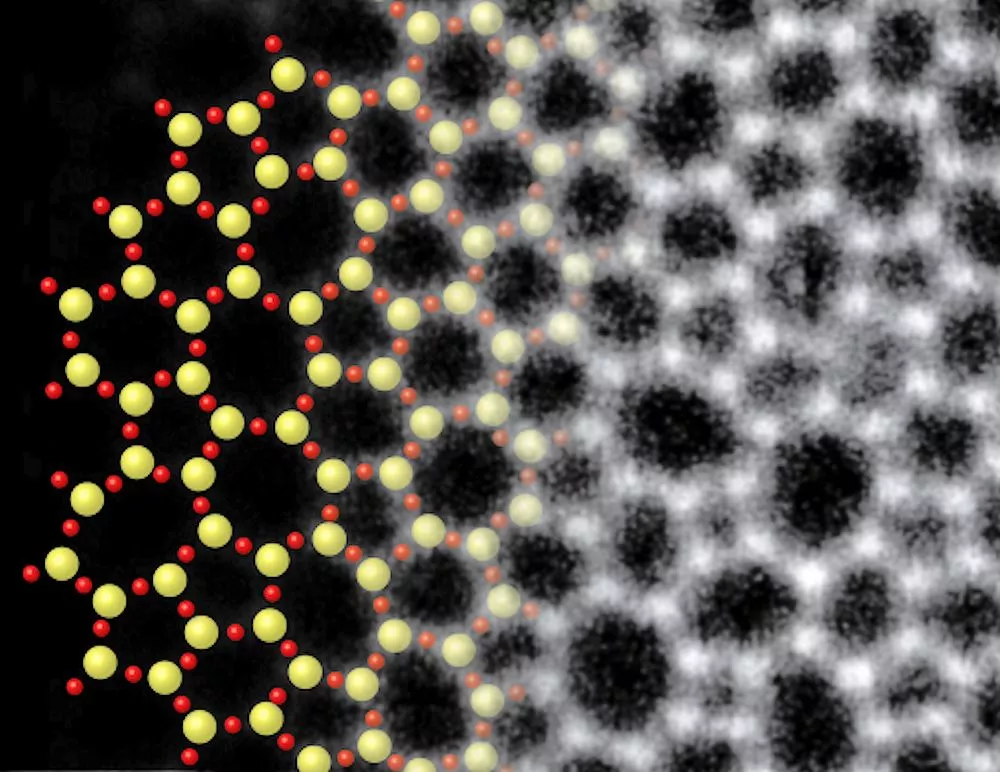
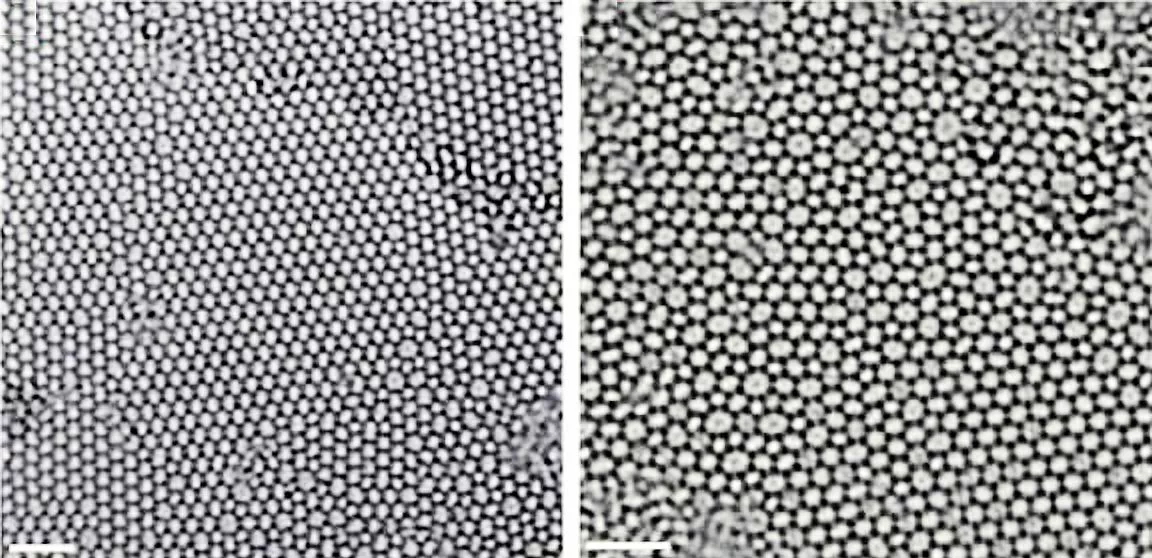
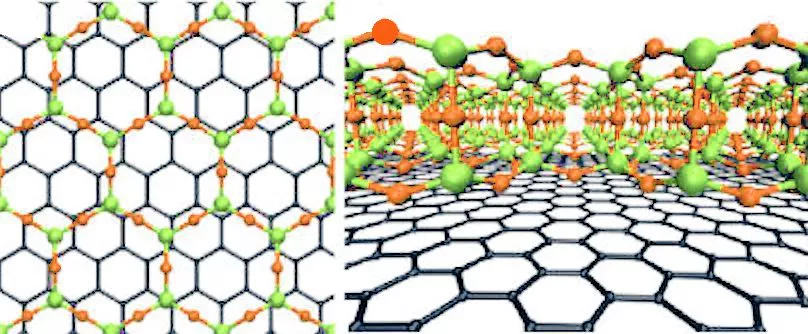
Electron microscopy showed that the atomic structure of the glass layer, grown atop about three layers of graphene, was in excellent agreement with glass structures predicted in 1932 by Prof. W.H. Zachariasen of the University of Chicago. The idea is that the chemical bonds in the glass are nearly the same as those in the crystalline form, but the basic SiO4 tetrahedra are linked together randomly.
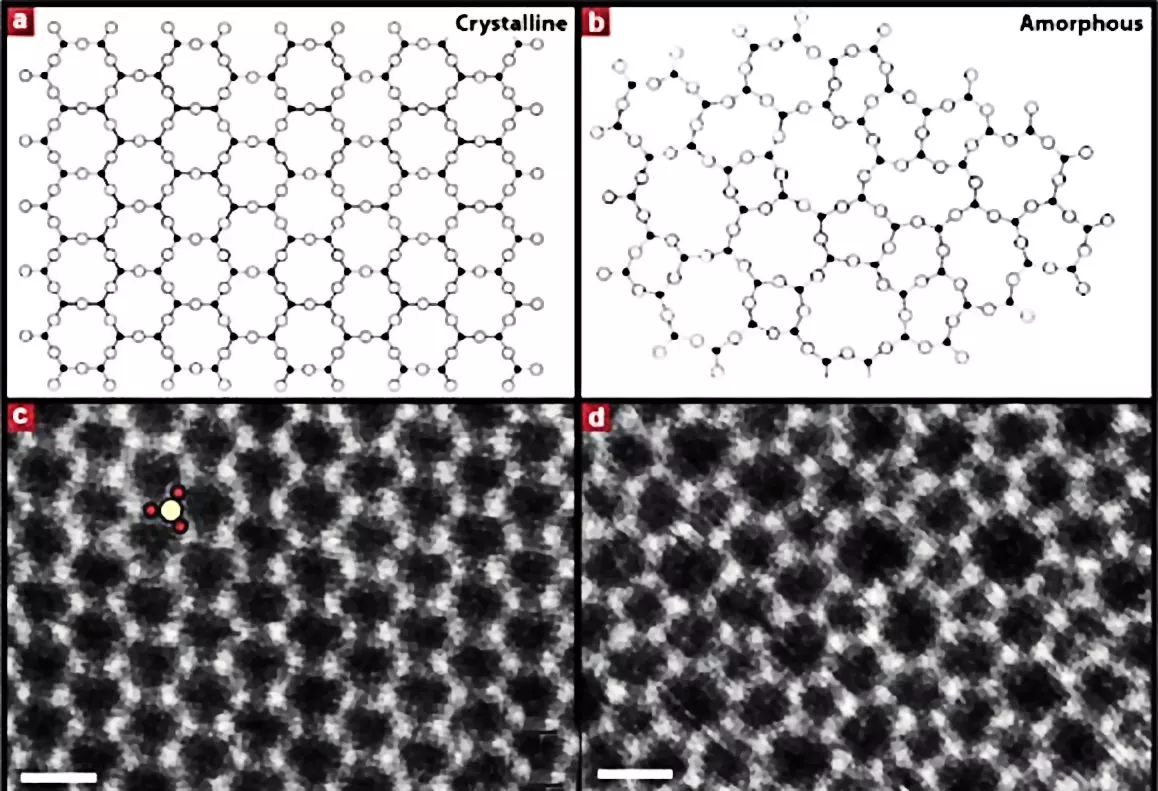
The figure above shows the structure of two-dimensional quartz on the left, and two-dimensional silica glass on the right. The black dots are the silicon atoms, and the open circles are oxygen atoms. While the crystalline quartz structure is composed entirely of six-member rings (six silicon atoms and six oxygen atoms), the silica glass has ring sizes ranging between about four and eight members, and even the six-member rings appear distorted.
Guinness may be most interested in this work as having produced the world's thinnest glass, but the lasting value will be in the direct atomic images that demonstrate that an inorganic glass has the sort of structure we always thought it had. This is not a surprise, but there is something satisfying about evidence in the form of an image – evidence that can be taken in at a glance. Prof. Muller's lucky creation has turned into a demonstration of physical law that will be well appreciated 100 years from now.
Source: Cornell University