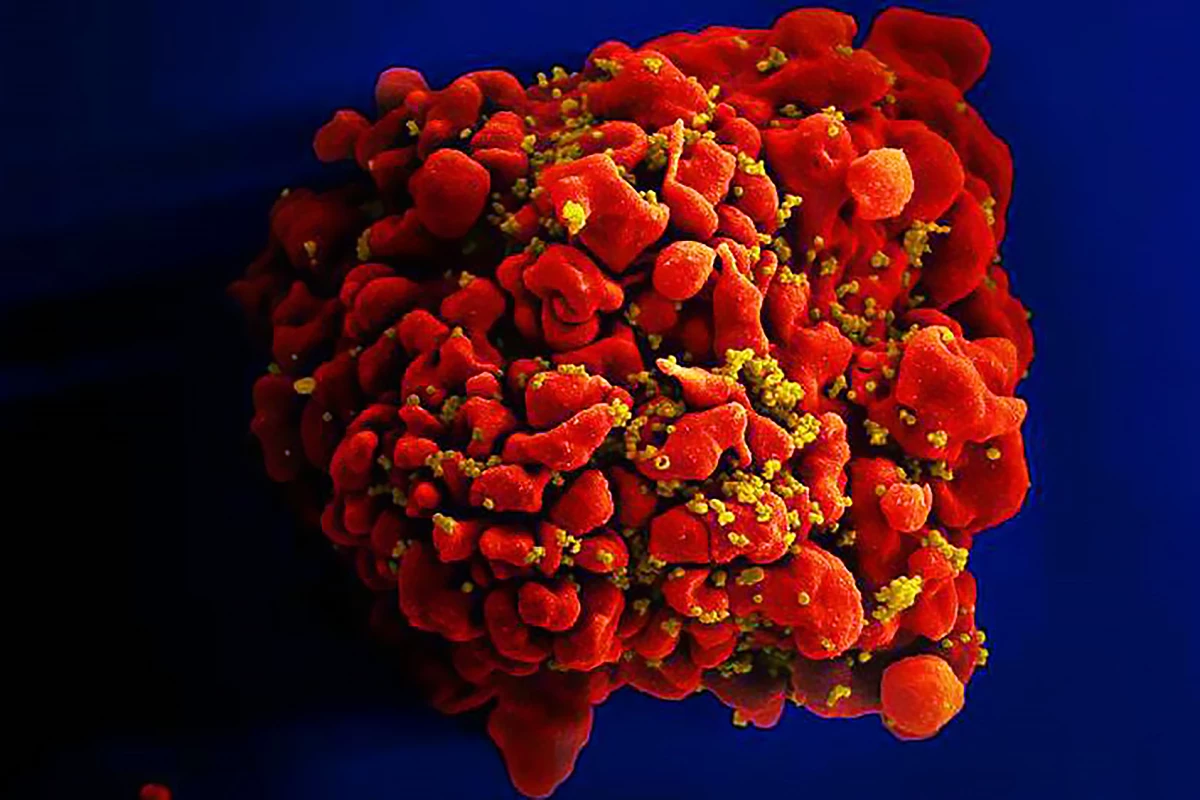
A groundbreaking discovery reveals how HIV integrates its genetic material into human DNA, exposing a key viral vulnerability that could lead to new therapies and bring the world closer to a functional HIV cure.
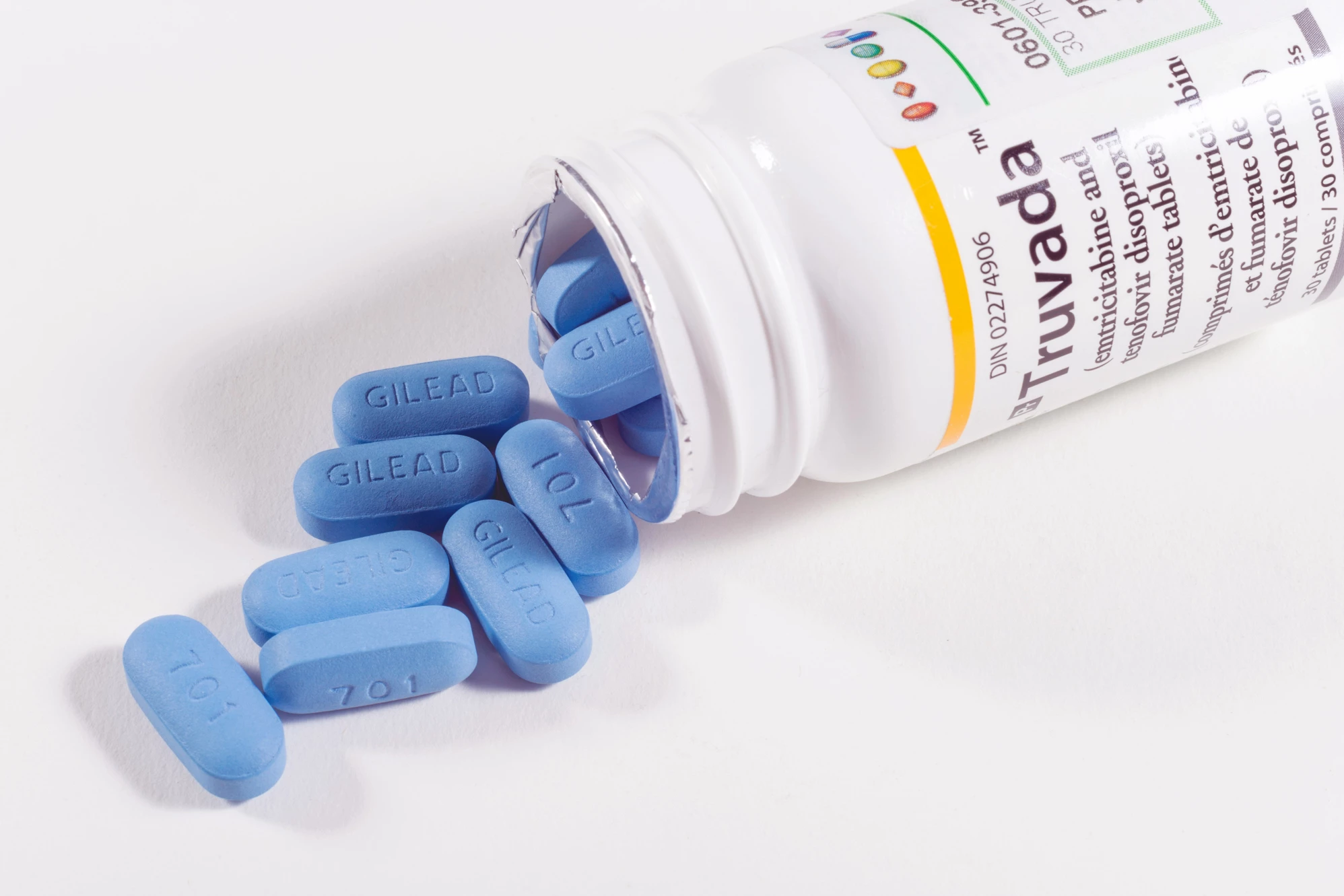
People with HIV can lead close-to-normal lives, thanks to antiretroviral therapy (ART) that prevents the virus from multiplying. The drawback, as with many medications, is that ART must be taken daily for life. Interruption to this regimen can reactivate hidden reservoirs of infected cells, leading to the rapid resurgence of HIV in a person’s blood and the development of drug-resistant variants.
New research by the German Center for Infection Research (DZIF) at Heidelberg University Hospital has identified a previously unknown mechanism by which the HIV-1 virus inserts, or “integrates,” its genetic material into human DNA after infection.
“Until now, it has not been entirely clear how HIV-1 integrase selects its integration targets in the genome,” said the study’s corresponding author, Marina Lusic, PhD, a group leader in DZIF’s Department of Infectious Diseases. “A deeper understanding of this process is crucial for developing new treatment strategies and tackling the persistent viral reservoirs that cannot be eliminated by existing therapies.”
By way of explanation, HIV carries an enzyme called integrase, which is kind of like a molecular “stapler.” Once the virus enters a human cell, it makes a copy of its genetic material in the form of DNA. Integrase’s job is to take that viral DNA and insert, or “staple,” it into the cell’s own DNA.
This step is crucial because, once HIV’s DNA is part of the cell’s genome, the virus can lie low and use the cell’s own machinery to make more copies of itself whenever it wants. It also means the infection becomes permanent: every time the cell divides, it copies the virus along with its own genes. Scientists have known for years that integration is essential to HIV infection, but what hasn’t been clear is how integrase decides where to insert the viral DNA. That’s what this new study shed light on.
The researchers used deep sequencing, which is a way of reading DNA very precisely, to identify the exact places where HIV’s DNA landed inside the host genome. They compared different patients and viral samples to see whether integration was random or followed a pattern. They also studied how the structure of human DNA, such as which parts are active and which are inactive, influences where HIV settles in.
They found that HIV doesn’t insert randomly. The virus has “preferences” for certain spots in the human genome, often inside active genes or areas where the DNA is more open and accessible. Where HIV lands affects how it behaves. If it lands in certain genes, it may give the virus an advantage, letting it persist longer in the body. Sometimes it integrates into spots that let infected cells grow and expand, creating long-lived reservoirs of infection that resist treatment.
The researchers found that certain “hotspots” of integration may help explain why HIV infection continues even under strong drug treatment. HIV doesn’t just look for open DNA; it also takes cues from nearby RNA molecules, which act like signposts that help guide integrase to specific spots in the genome.
“This discovery opens a new avenue for HIV intervention,” Lusic said. “If we can disrupt the virus’s ability to use host RNA structures for integration, we may be able to limit or redirect where HIV hides and ultimately reduce or eliminate the need for lifelong therapy.
“These findings are particularly significant in light of the increasing global instability in HIV care. In many regions, the continuous provision of antiretroviral therapies is not guaranteed – with the result that interruptions significantly increase the risk of treatment failure and the spread of resistant virus variants.”
The study was published in the journal Nature Microbiology.
Source: DZIF
New Atlas may receive commission for purchases made via links.