Researchers have identified a previously unknown biological process that causes tissue and organ damage in conditions where oxygen is low, such as heart attacks and strokes. The study suggests that bursting red blood cells, not blood clots, are the culprits.
The microvasculature, the body’s network of tiny blood vessels, is critical for delivering oxygen and nutrients to tissues. Damage to these vessels can contribute to life-threatening conditions like heart attack and stroke. In these conditions, microvascular dysfunction leads to poor blood flow, lack of oxygen, tissue death, and inflammation, all of which can worsen outcomes.
A new study by a team of researchers from institutes in Australia and New York has identified a previously unknown biological process that causes tissue and organ damage in low-oxygen conditions. It’s driven by red blood cells and not the blood clots traditionally associated with this sort of damage.
“We’ve discovered a completely new blood-clotting mechanism that has nothing to do with the traditional clotting system involving platelets or fibrin,” said corresponding author Professor Shaun Jackson, founder and director of ThromBio, a drug discovery company focused on developing anti-clot medications. “Instead, dying cells cause red blood cells to burst and their membranes act like a biological glue – sealing off damaged blood vessels and blocking blood flow to vital organs.”
According to the traditional clotting pathway, when a blood vessel is injured, tiny cell fragments called platelets quickly stick to the damaged site and one another, forming a temporary plug. At the same time, a cascade of proteins in the blood activates the protein fibrin, which forms a mesh that stabilizes the platelet plug, creating a strong, lasting clot to seal the vessel and stop bleeding.
The researchers were aware that both acute and long COVID infections can damage the body’s tiny blood vessels, making it harder for the blood to circulate properly. It was initially thought that excessive fibrin was the culprit, but treating patients with blood thinners to clear the fibrin was of little benefit. So, the researchers looked for another cause.
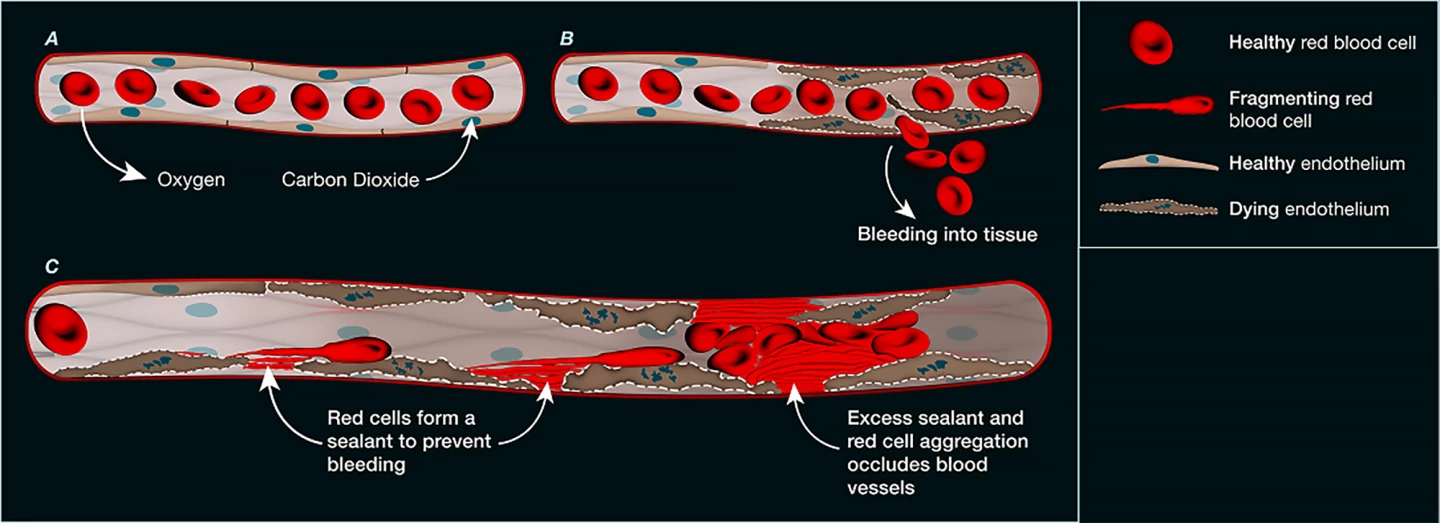
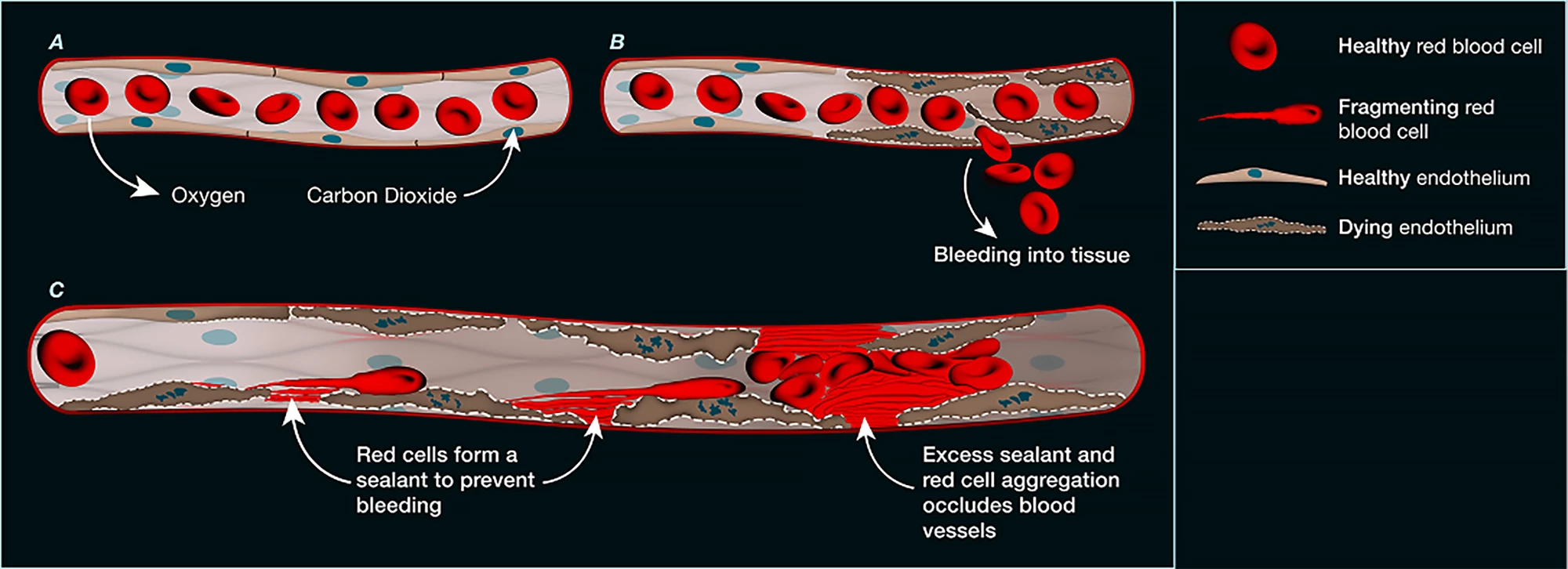
Examining more than 1,000 blood vessels taken from deceased patients with COVID-19, they found that the endothelial cells that form the inner lining of blood vessels were damaged. The damage was widespread, evident in the small vessels of the lungs, heart, kidneys, and liver, and it appeared that the endothelial cells in those vessels had died. Under the microscope, the researchers could see deposits of a protein-like material at the sites of endothelial cell death. Upon closer inspection, the material was found to have originated from ruptured (hemolyzed) red blood cells, whose sticky contents had spewed out and clogged the microvasculature.
The researchers found that this new form of microvascular blockage was not only seen in COVID-19 patients. Using mouse models, they confirmed it also occurred after heart attack, stroke, and gut ischemia, which occurs when the blood flow to the intestines slows or stops, and they’re starved of oxygen and nutrients.
“This mechanism helps explain why many patients with severe COVID or other critical illnesses suffer from multiple organ failure, even when clotting is under control,” Jackson said. “It’s a whole new chapter in vascular biology.”
The discovery has obvious implications for medical treatment. As has been mentioned, currently used blood thinners (anticoagulants) don’t work well in microvascular COVID-19 because blood clots aren’t the main problem.
“Rather than targeting platelets or clots, therapies might instead aim to prevent endothelial cell death or block the red blood cell damage that follows,” said Jackson. “By stopping this process early, we may be able to preserve blood flow, protect organs and ultimately save lives.”
The study was published in the journal Nature.