As the engine room of so much of the modern world, there is an intense focus on boosting the performance of today’s batteries and researchers are exploring all kinds of avenues in pursuit of that aim. Whether it is crafting the world’s fastest electrodes, building battery parts out of nuclear waste or preventing fire danger with the help of sound waves, 2020 showed us just how imaginative scientists can be when it comes to developing technologies for next-generation energy storage.
This year, we’ve looked at numerous creative ways to improve the functionality of electrodes, seen how dashes of graphene can make electrolytes tougher and how advanced materials might help batteries charge faster or offer far greater densities. Let’s have a look at these research highlights, along with some more radical battery designs that are the result of thinking outside the box.
Putting the pedal to the metal

When it comes to boosting batteries by bringing in new materials, all options are on the table, but one with huge potential is lithium metal. Described by some as a “dream material,” using lithium-metal as the anode in place of the graphite and copper currently used could significantly boost the density of today’s batteries, enabling them to run far longer and hold far more energy.
The problem is safety. As the battery is charged, growths known as dendrites tend to form on the surface of the lithium metal anode, causing electrical shorts, fires and ultimately the failure of the device. We saw a few innovative approaches to solving this problem in 2020, including one from scientists at Washington State University whose approach to preventing dendrites involved adding a few key chemicals to the cathode and electrolyte solution.
This led to the formation of a protective layer on the surface of the lithium metal anode, enabling it to remain stable while being charged across 500 cycles. Working in the team’s favor as it eyes commercialization is that the process can be integrated into existing manufacturing procedures.
Solid state, no dendrites
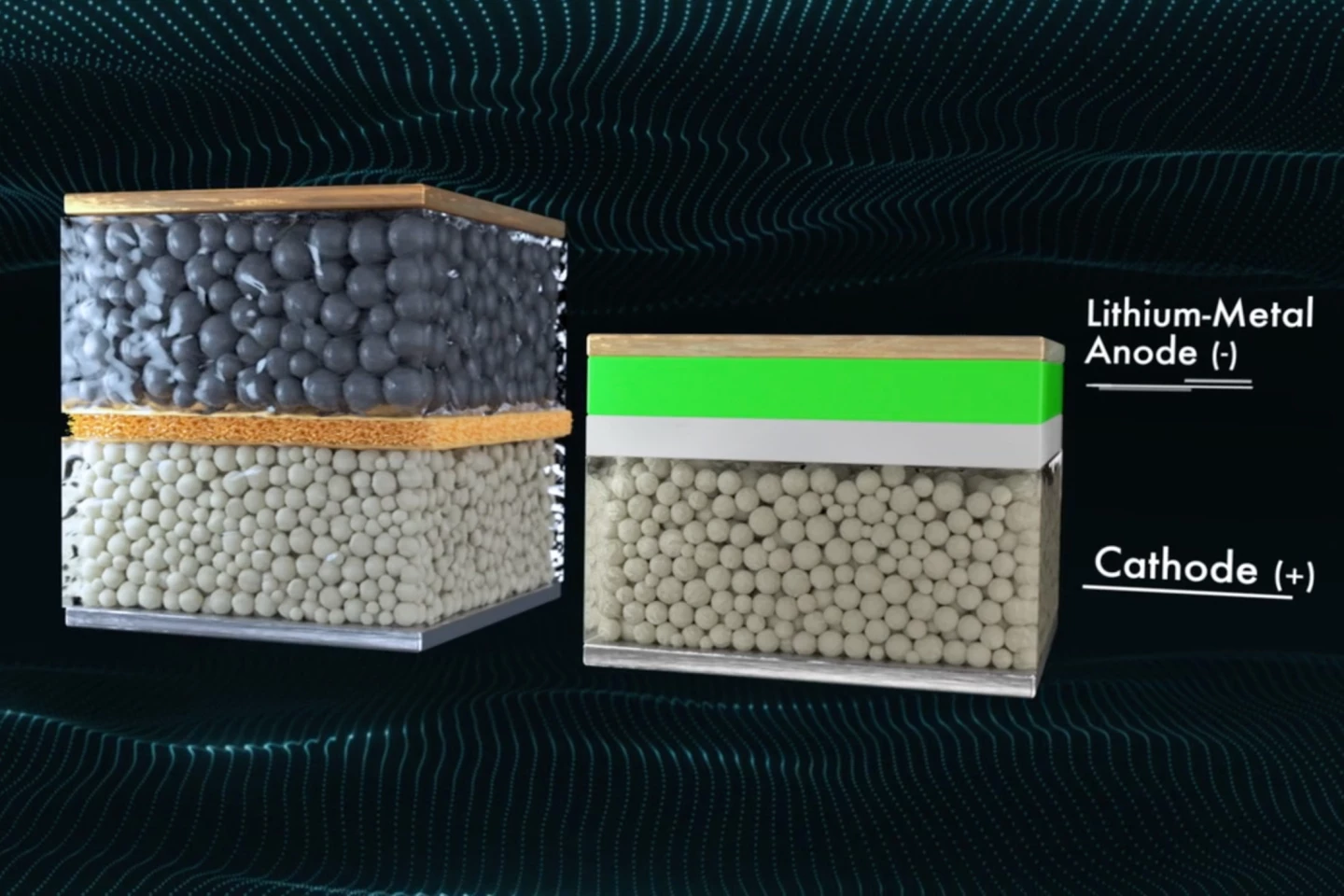
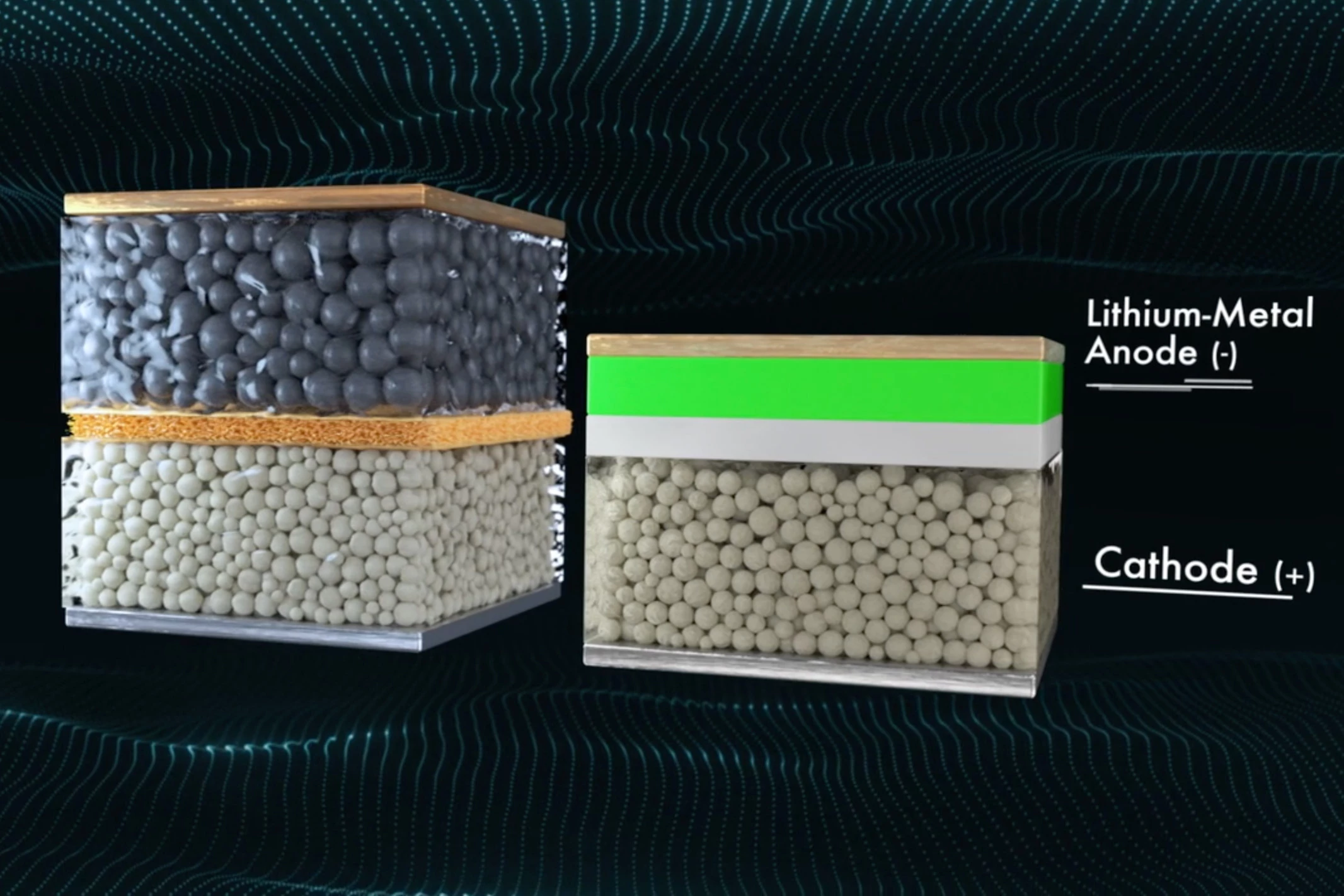
In December, Californian battery-maker QuantumScape announced some performance numbers for its solid-state lithium-metal battery designed for use in electric vehicles, and they certainly caught our attention. Could a flat electric car one day be charged to as much as 80 percent in as little as 15 minutes?
The company claims that indeed it could, thanks in part to the use of a solid electrolyte rather than a liquid one, and an anode made from lithium metal that forms itself around the current collector as the battery is charged. This solid state lithium-metal battery also avoids the dendrite problem thanks to a separator between the anode and cathode made from solid ceramic material.
The upshot of this innovative design is a battery with excellent energy density, around four times that of the lithium batteries in Tesla’s Model 3 at 1 kWh/liter in volumetric terms. When it comes to weight, the battery also offers between 380 and 500 wH per kg, which is over and above the 260 Wh per kg in Tesla’s battery packs. The battery was also found to retain 80 percent of its capacity after 800 cycles, which bodes well for its safety and longevity.
Riding the wave

In February, we looked at a rather imaginative approach to preventing dendrite growth in lithium-metal batteries, from a group of researchers at University of California San Diego. The team built a tiny ultrasound device and incorporated it into a lithium-metal battery, enabling it to send high-frequency sound waves through the liquid electrolyte as a way of causing it to flow gently rather than remain static.
This had the effect of creating neat, uniform distribution of lithium on the anode rather than the uneven clumps that usually form and lead to dendritic growth. In testing, this ultra-sound equipped battery could be charged from zero to 100 percent in just 10 minutes, and proved stable across 250 charging cycles, again boding well for safety.
A fast-charging battery

In yet another example of how scientists may make lithium metal batteries a reality, scientists at Texas A&M University showed off a device that incorporates tiny scaffolds made of carbon nanotubes as the anode. These are laced with molecules that cause lithium ions to bind to the surface, helping avoid the formation of dendrites on the surface.
While this design ticked the boxes in terms of safety, this battery architecture also proved capable of producing larger currents. So much larger, in fact, that the team reported that its device could handle currents five times that of conventional batteries, raising the prospect of one that can be charged in just a fraction of the time.
Bringing silicon into the mix

While lithium metal shapes as an anode material with plenty of potential, there are other exciting possibilities on the horizon. Silicon is one of example, with an ability to store four times the amount of lithium ions of today’s graphite and copper, but the capacity tends to decline rapidly.
Back in June we looked at a potential solution to this problem, where scientists at the Korea Institute of Science and Technology used a technique called lithium pre-loading to improve the device’s longevity. This involved submerging the silicon anode in a special solution that causes electrons and lithium ions to seep into the electrode, to make up for the losses that occur during cycling.
Where most silicon-based anodes shed more than 20 percent of their lithium ions during the initial charging cycle, this new anode lost less than one percent during testing. It was also shown to have an energy density 25 percent higher than a commercially available counterparts.
Microwaves and salt

Another battery chemistry with plenty of potential, but for very different reasons, is sodium-ion. Lithium is relatively rare and mining it can be costly and damaging to the environment. Salt on the other hand, is everywhere and this abundance could translate into far cheaper batteries for large, grid scale applications. In April we saw how a key component of these batteries could be sourced from a similarly abundant material.
Starting with recyclable PET plastic, scientists at Purdue University were able to reduce the material to flakes, which were then treated with ultrafast microwave irradiation to turn them into something known as disodium terephthalate. This small organic molecule has long been fancied as a potential anode material due to its excellent electrochemical performance, and the team was able to demonstrate its creation as part of a functioning sodium-ion cell.
“We are helping to address the growth in the proliferation of renewable energy conversion and storage, which stems from the societal attention and increasing awareness of climate change and energy resource limitation," lead researcher Vilas Pol said at the time.
Inspiration from the seas

Another alternative battery design that researchers hope could come to offer grid-scale storage solutions for renewable energy is the redox flow battery. Instead of storing energy inside the battery itself, these devices hold their energy in liquid electrolytes in huge external tanks, which means the storage potential can simply be increased by increasing the size of the tanks.
In June, an MIT research team demonstrated how a key building block of these batteries could also be made from more sustainable materials. Chitin is a cellulose-like polysaccharide found in shrimp shells, and the researchers were able to use it in combination with felt to produce electrodes for a redox flow battery with a superior power density.
"Its benefit does not only lies in its good performance, but also in the low cost of the starting material, which makes the electrodes more sustainable, given the reuse of waste," said Francisco Martin-Martinez, senior author of the study.
Making gravity our friend

Another promising solution to large-scale storage of renewable energy could lie in the forces of gravity. Scottish company Gravitricity is pursuing a novel energy storage system consisting of massive weights, and powerful winches and cables that hold them aloft. When energy is needed, these weights can be dropped down a shaft which turns the winch and generates electricity.
This can take place in as little as 15 minutes, or draw out the power release over up to eight hours, for peak outputs between 1 and 20 MW. This shapes as a low-cost and long term energy solution, and Gravitricity is pressing ahead with construction of a prototype system, which will be tested out on completion in Edinburgh sometime in late 2021.
A touch of graphene for toughness

In June we looked at another example of a solid state battery, one with an impressive degree of durability. These devices swap out the liquid electrolyte for a solid one in hopes of achieving greater energy densities, but often this can lead to fracturing or corrosion of the battery.
A team of Brown University researchers sought a solution to this by turning to the wonder material graphene, which was added in small amounts to ceramic materials to form a solid electrolyte they claimed to be the toughest that anyone has ever made.
What's interesting about this bit of research is that graphene is highly conductive of electricity, which is not a desirable attribute for a battery electrolyte, which should conduct ions instead. But by keeping the concentration of graphene low enough, the team was able to find a sweet spot that prevented it from conducting electricity, but still offered supreme strength.
The world's fastest electrodes

All batteries contain a pair of electrodes, the cathode and anode, through which electrical current flows, and these are typically messy structures that require the charge-carrying ions to navigate a tangled, spaghetti-like maze on the way in and out. Ultracapacitor-maker Nawa introduced its own version of an electrode in October that offers a much more straightforward path.
The electrode consists of a vertically aligned structure resembling that of a hairbrush, with a hundred billion highly conductive carbon nanotubes sitting bolt upright and coated in active materials, such as lithium-ion. This basically creates an expressway for the traveling ions, enabling them to travel in and out of the battery in far more expedient fashion.
In practical terms, the company says its lightning-fast electrodes could increase the charge and discharge rates of a battery by ten times, possibly enabling a 0-80 percent charge in as little as five minutes. Energy density, meanwhile, could jump by a factor or two or three.
Nawa says its manufacturing process for these electrodes is cheap, and its confident it will be cost-competitive with existing electrodes. It expects the technology to be begin to enter the market in simpler forms in 2022, and then more advanced versions from 2023 onwards. It is currently in talks with a number of car companies to that end, and has lined up its first large-scale customer in French battery maker Saft.