Two promising avenues in the development of next-energy storage involve the use of high-density lithium metal and an electrolyte that is solid rather than liquid, and a new study brings these branches of battery research together in an exciting new breakthrough. Scientists in the US have demonstrated how stability issues associated with these architectures might be solved with the help of electrochemical pulses, paving the way for electric vehicles and smartphones that run for far longer on each charge.
Part of this field of research focuses on anodes, which act as one of the device's two electrodes and helps facilitates the transport of lithium ions via a liquid electrolyte. Today's anodes are made from a mix of graphite and copper, but pure lithium metal is a tantalizing alternative as it offers the highest energy density among solid materials. Integrating lithium metal into batteries has proven difficult so far, however, with scientists running into various safety issues that swiftly bring them undone.
There is a line of thinking that using a solid electrolyte in place of a liquid one would lead to a battery better suited for use with lithium metal. And this intersection of materials is the focus of the new work from scientists at Oak Ridge National Laboratory (ORNL), who believe they've come up with a way to join them together in a stable and long-lasting way that doesn't compromise performance.
Melding materials together in solid-state batteries is typically a tricky task, as ongoing charge and discharge cycles leads to instability in the joints and cause voids to form, something known as contact impedance. Applying pressure is one way this problem might be solved, but is a technique that would need to be used periodically as the battery is operated, and can also cause it to short.
The ORNL scientists found that they could eliminate these voids by applying a short, high-voltage electrochemical pulse when the lithium metal anode is joined with a solid electrolyte. These pulses see a current surround the voids that causes them to dissipate, resulting in more widespread contact at the interface of the materials.
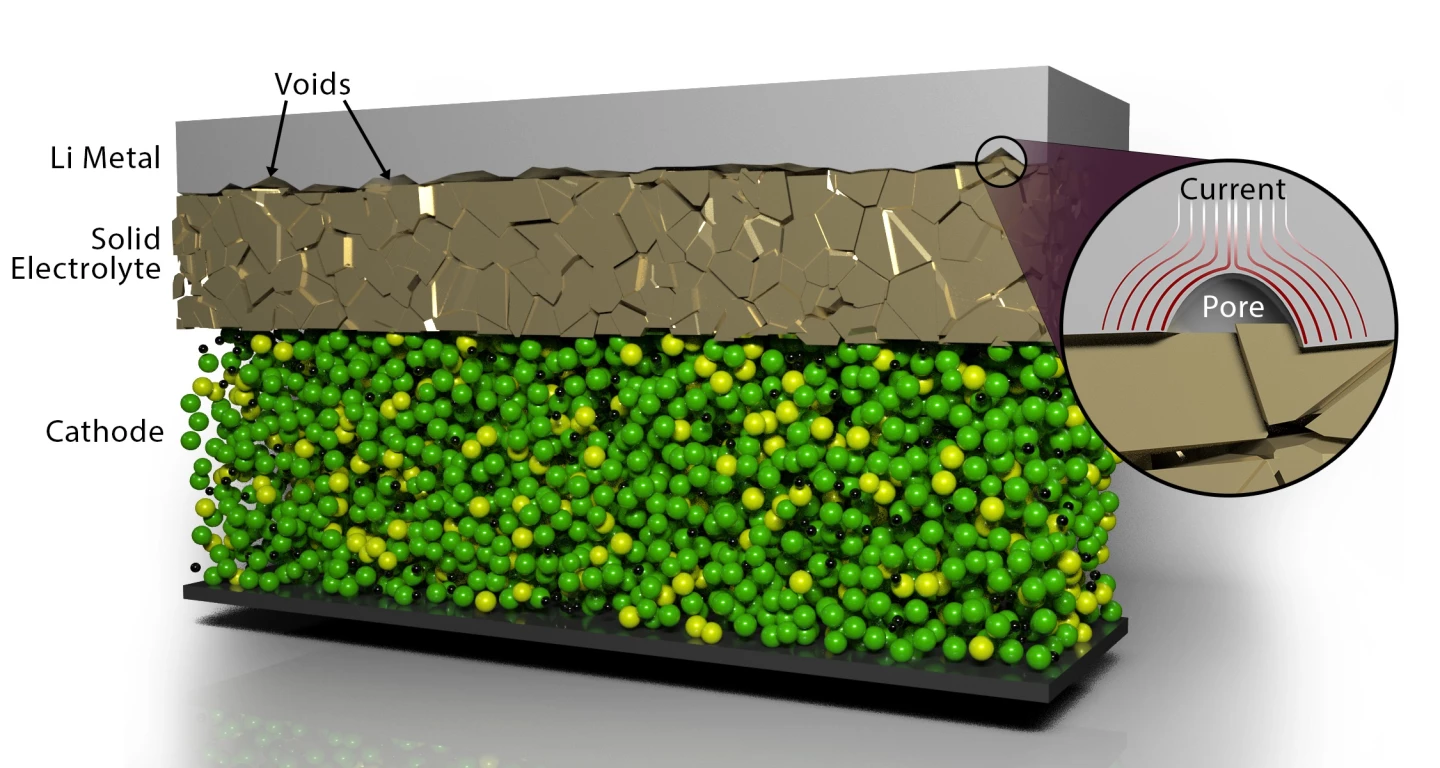
Because this has no detrimental effect on the battery, and the pulsing technique could be applied to restore it to nearly its original capacity, the scientists imagine this technology one day offering a viable way to manage solid-state, lithium-metal batteries during operation. They say this kind of system could offer twice the energy density of today's solutions in a much smaller package, which would mean electric vehicles can travel much farther per charge, or smartphones that run for days at a time.
“This method will enable an all-solid-state architecture without applying an extrinsic force that can damage the cell and is not practical to deploy during the battery’s usage,” said Ilias Belharouak, co-lead on the project. “In the process we’ve developed, the battery can be manufactured as normal and then a pulse can be applied to rejuvenate and refresh the interface if the battery becomes fatigued.”
The scientists are now continuing to develop the technology, by experimenting with more advanced electrolyte materials, and investigating how it might be scaled up for use in a working-scale solid-state battery system.
The research was published in the journal ACS Energy Letters.
Source: Oak Ridge National Laboratory






