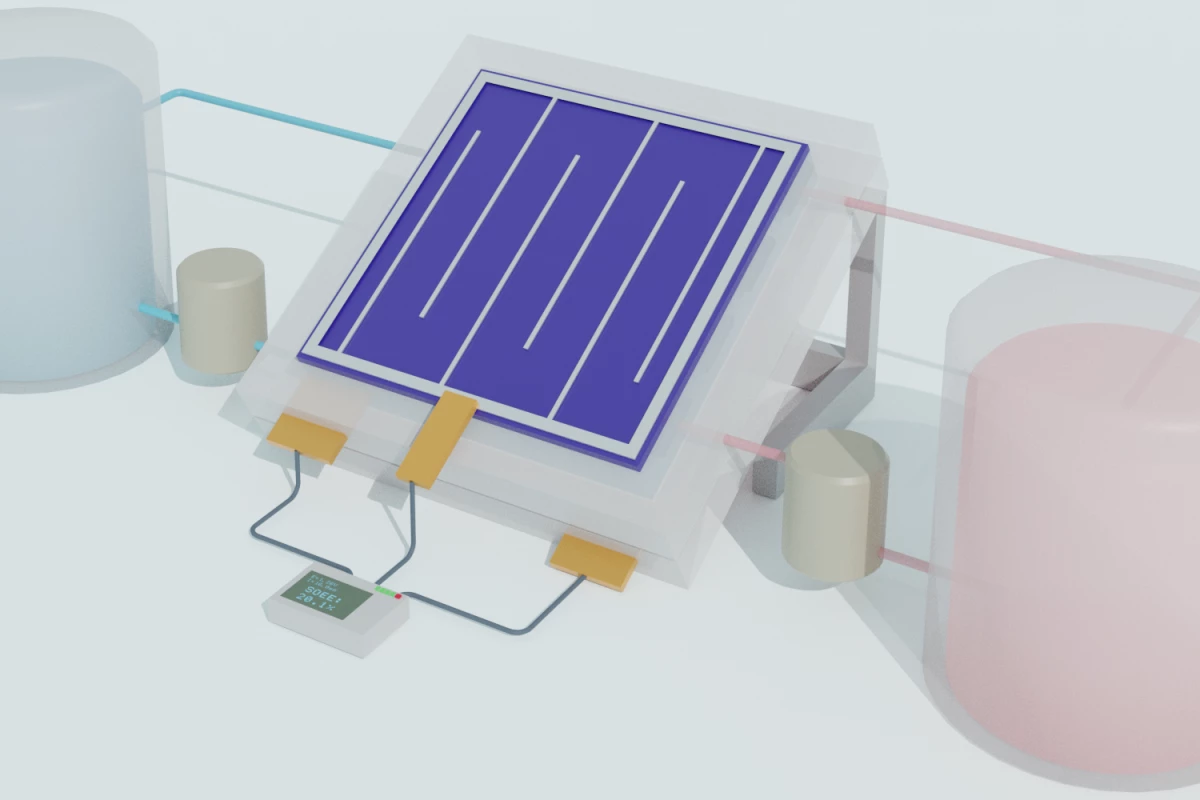
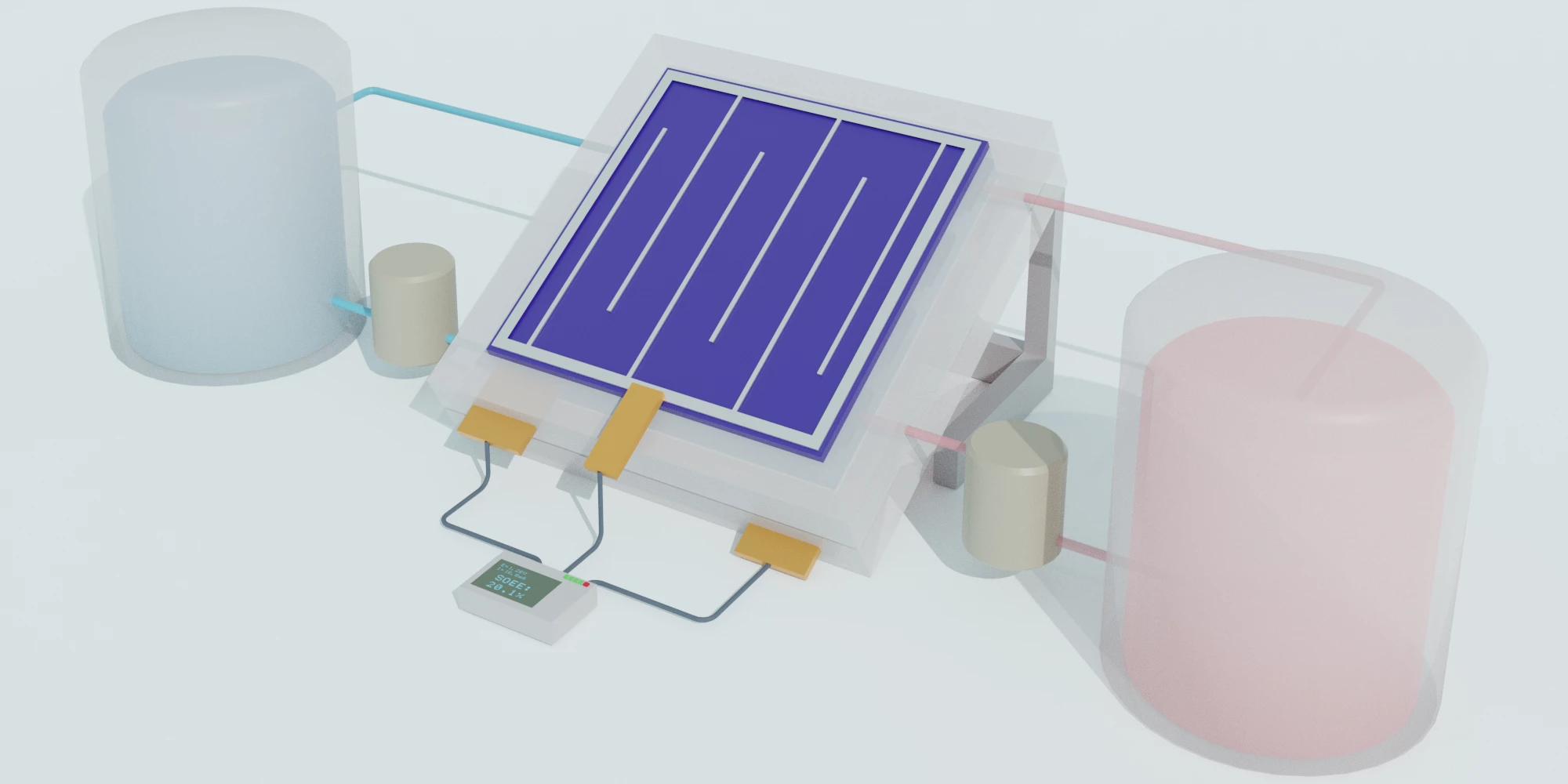
Capturing energy from the Sun with solar panels is only half the story – that energy needs to be stored somewhere for later use. In the case of flow batteries, storage is relegated to vats of liquid. Now, an international team led by University of Wisconsin-Madison scientists has created a new version of these solar flow batteries that’s efficient and long-lasting.
To make the new device, the team combined several existing technologies. It’s a silicon/perovskite tandem solar cell, paired with a redox flow battery, which the team says will allow people to harvest and store renewable energy in one device. Not only is it efficient, but it should be inexpensive and simple enough to scale up for home use.
The energy-harvesting part of the equation combines the long-time industry-leading material – silicon – with a promising young upstart called perovskite. These tandem solar cells have proved better than either material alone, since the two materials capture different wavelengths of light.
For storage, the team turned to a flow battery. Traditionally, these devices contain two liquids, housed in separate tanks, that function as the electrolytes. Electricity from the solar cell charges one of the liquids, where it can sit more or less indefinitely. When the power is needed, the two liquids interact in a middle chamber, creating a chemical reaction that produces electricity.

The team used a theoretical modeling method to determine which chemicals would operate at the ideal voltage, to maximize efficiency. They settled on two organic compounds dissolved in saltwater, and tests with the final physical device confirmed that it was a good match.
The team recorded 20 percent efficiency, which is up there with the best. The device was able to maintain a high efficiency, and most of its capacity, over hundreds of hours and charge-discharge cycles. That gives it a much longer life than other flow batteries, whose acidic electrolytes tend to corrode the tanks.
“That’s 20 percent efficiency any time you like,” says Song Jin, lead researcher on the study. “You can use the solar electricity right away during the day and get 20 percent, or you can use it in the evening from storage and get 20 percent.”
The team plans to continue developing the solar flow batteries to improve efficiency, reduce costs and investigate ways to scale them up for practical large-scale use.
The research was published in the journal Nature Materials.
Source: University of Wisconsin-Madison