One particularly promising architecture for next-generation batteries uses pure lithium-metal, a material with excellent energy density that could see electric vehicles travel many times farther on each charge. A research group in the US has taken a significant step forward with this technology, coming up with a design for a long-lasting lithium metal battery that remains functional for a record-breaking number of charging cycles.
The idea behind these types of batteries is to swap out the graphite used in the anode component for pure lithium metal, which can hold as much as 10 times the energy. Described as a dream material by some researchers, lithium metal is seen as key to helping us break through a key bottleneck in energy storage, but scientists have struggled with longevity issues with the versions developed so far failing quickly during use.
One of the reasons for that failure is the complex reactions that occur around the anode, affecting a thin film on top of it known as the solid electrolyte interphase (SEI). This film controls the molecules that enter the anode from the electrolyte solution, through which electrons travel back and forth to and from the battery's other electrode, known as the cathode.
In this way, this gatekeeping role tasks the SEI with the responsibility of preventing unwanted chemical reactions as the battery is cycled, and this is the mechanism targeted by the scientists from the U.S. Department of Energy’s Pacific Northwest National Laboratory (PNNL) in a new study. It is commonly thought that loading up the amount of lithium in the anode was one way to address this problem, but the team found success through another approach.
“Many people have thought that thicker lithium would enable the battery to cycle longer,” says Jie Xiao, a corresponding author of the paper. “But that is not always true. There is an optimized thickness for each lithium-metal battery depending on its cell energy and design.”
The scientists used very thin strips of lithium as the basis for their anode, each with a width of just 20 microns, far thinner than a human hair. This anode was worked into a pouch cell battery with an energy density of 350 Wh/kg. The best-in-class lithium-ion batteries in use today have a density of 250 to 300 Wh/kg, so while 350 Wh/kg isn't unheard of in research circles, it would be a marked improvement on currently available technologies.
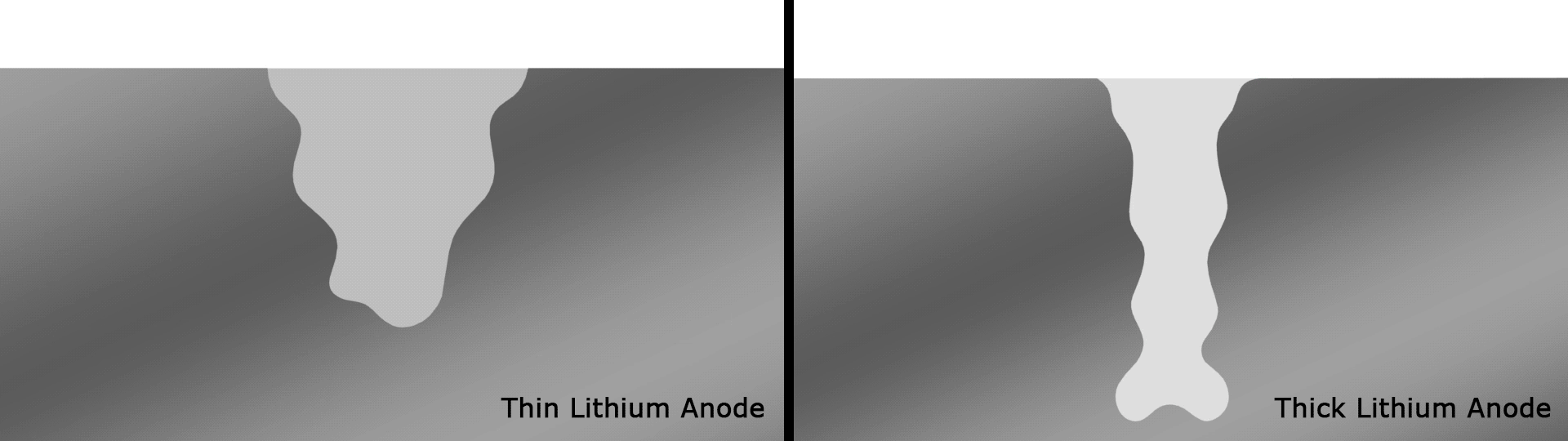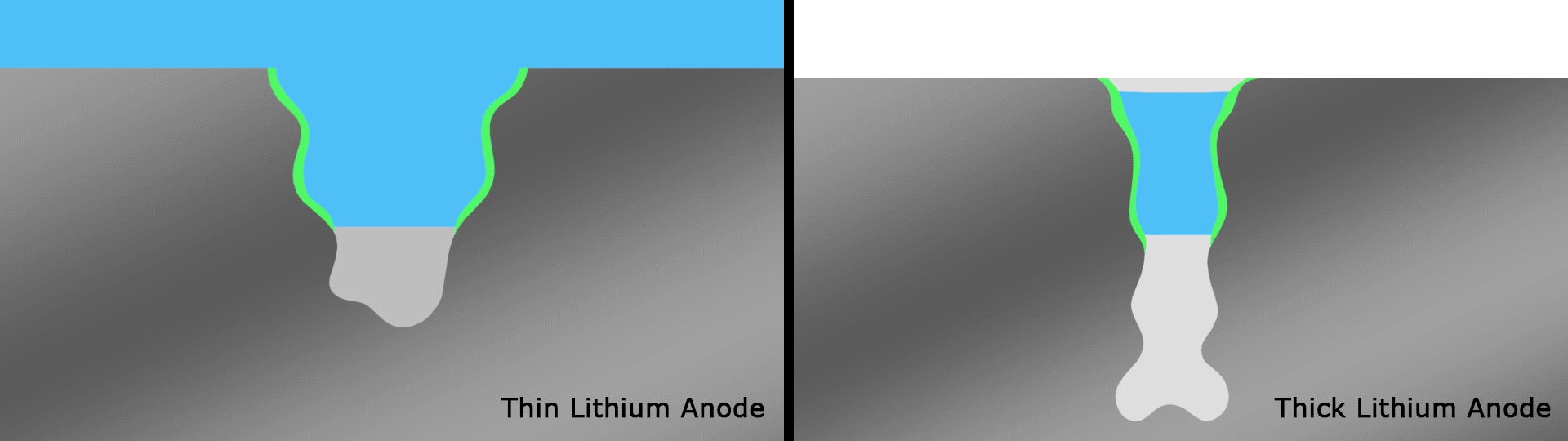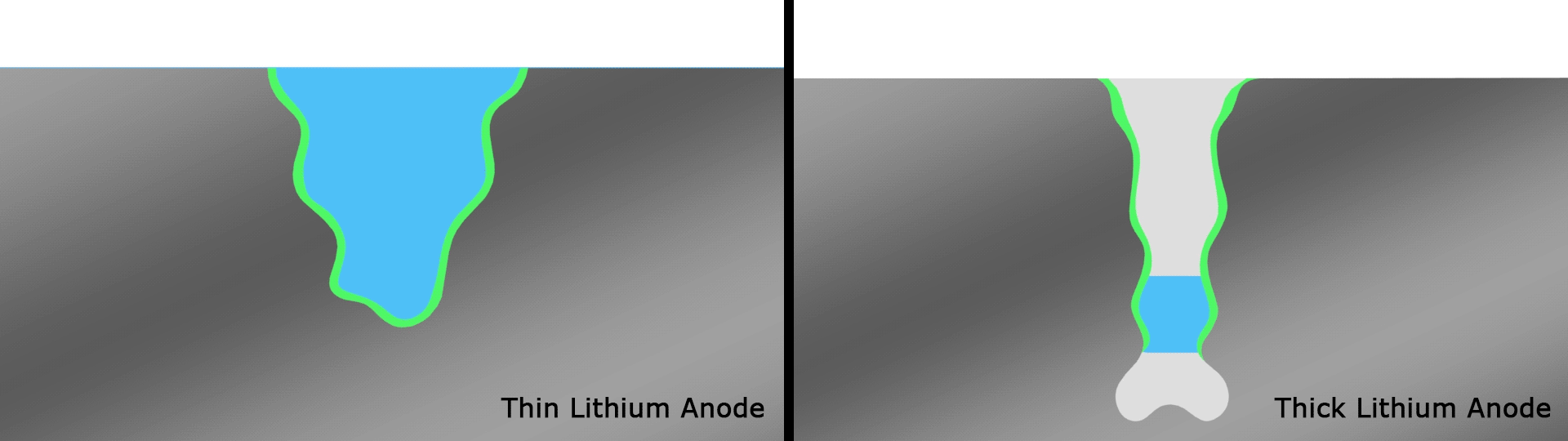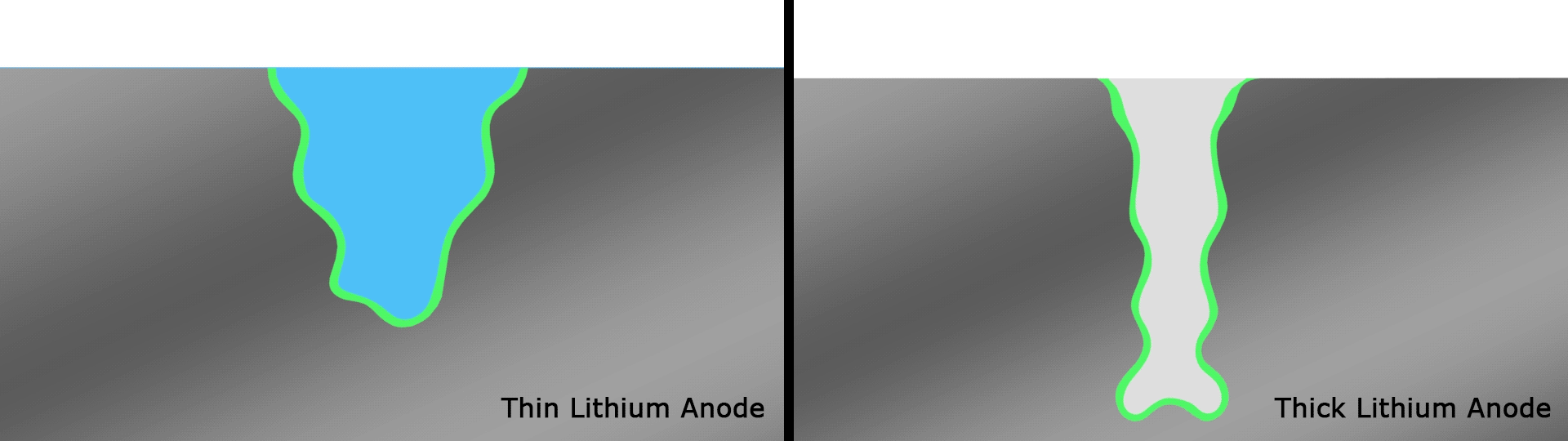
In testing, the team found that the battery retained 76 percent of its capacity after a record 600 cycles. The same researchers had demonstrated an experimental lithium-battery that could operate across 50 cycles four years ago, then one capable of 200 cycles two years ago. This current record-setting version is another key step forward and, according to the team, lasts far longer than any others under development in similar research projects.
The team attributes the success of the design to the way the thinner strips facilitate a better SEI and therefore better interactions between the electrolyte and anode, compared to thicker strips that smother important electrochemical reactions. Having solved a key issue relating to lithium metal batteries, the authors are hoping to continue to improve the technology through a multi-institute consortium known as Battery500, which is working towards an energy density of 500 Wh/kg.
“The Battery500 Consortium has made great progress in increasing the energy density and extending the cycle life,” says Stanley Whittingham, 2019 Nobel Prize laureate in chemistry and a coauthor of the paper. “But much more needs to be done. In particular, there are safety issues with lithium-metal batteries that must be addressed. That’s something that the Battery500 team is working hard to resolve.”
The research was published in the journal Nature Energy.





