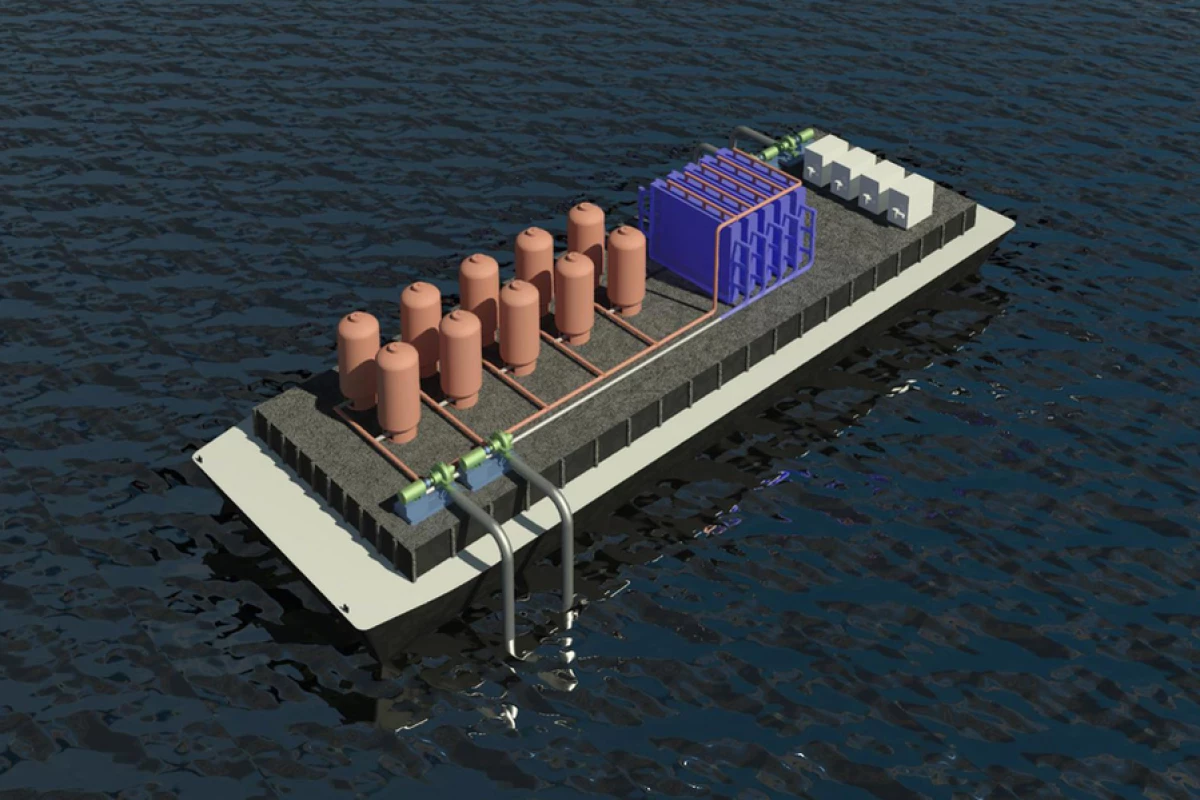
Pulling greenhouse gases out of water is an odd-sounding idea, but the oceans are the planet's number one carbon sink, and direct air carbon capture has pretty serious problems: it costs a lot, and uses a lot of energy. According to IEA figures from 2022, even the more efficient air capture technologies require about 6.6 gigajoules of energy, or 1.83 megawatt-hours per ton of carbon dioxide captured.
Most of that energy isn't used to directly separate the CO2 from the air, it's in heat energy to keep the absorbers at operating temperatures, or electrical energy used to compress large amounts of air to the point where the capture operation can be done efficiently. But either way, the costs are out of control, with 2030 price estimates per ton ranging between US$300-$1,000. According to Statista, there's not a nation on Earth currently willing to tax carbon emitters even half of the lower estimate; first-placed Uruguay taxes it at US$137/ton. Direct air capture is not going to work as a business unless its costs come way down.
It turns out there's another option: seawater. As atmospheric carbon concentrations rise, carbon dioxide begins to dissolve into seawater. The ocean currently soaks up some 30-40% of all humanity's annual carbon emissions, and maintains a constant free exchange with the air. Suck the carbon out of the seawater, and it'll suck more out of the air to re-balance the concentrations. Best of all, the concentration of carbon dioxide in seawater is more than 100 times greater than in air.
Previous research teams have managed to release CO2 from seawater and capture it, but their methods have required expensive membranes and a constant supply of chemicals to keep the reactions going. MIT's team, on the other hand, has announced the successful testing of a system that uses neither, and requires vastly less energy than air capture methods.
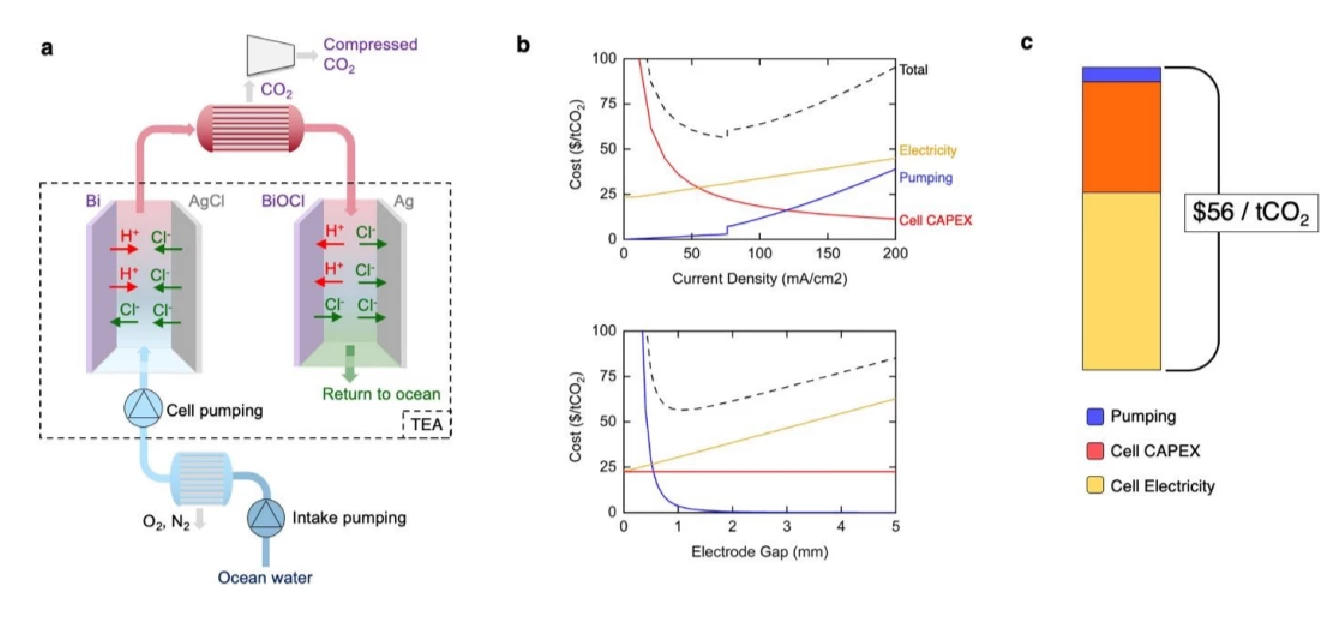
In the new system, seawater is passed through two chambers. The first uses reactive electrodes to release protons into the seawater, which acidifies the water, turning dissolved inorganic bicarbonates into carbon dioxide gas, which bubbles out and is collected using a vacuum. Then the water's pushed through to a second set of cells with a reversed voltage, calling those protons back in and turning the acidic water back to alkaline before releasing it back into the sea. Periodically, when the active electrode is depleted of protons, the polarity of the voltage is reversed, and the same reaction continues with water flowing in the opposite direction.
In a new study published in the peer-reviewed journal Energy & Environmental Science, the team says its technique requires an energy input of 122 kJ/mol, equating by our math to 0.77 mWh per ton. And the team is confident it can do even better: "Though our base energy consumption of 122 kJ/mol-CO2 is a record-low," reads the study, "it may still be substantially decreased towards the thermodynamic limit of 32 kJ/mol-CO2."
The team projects an optimized cost around US$56 per ton of CO2 captured – although it's not fair to compare that directly against full-system direct air capture costs. The study cautions that this does not include vacuum degassing, filtration and "auxiliary costs outside of the electrochemical system" – analyses of which will have to be done separately. Some of these, however, could potentially be mitigated by integrating the carbon capture units in with other facilities, for example desalination plants, which are already processing large volumes of seawater.
There are some other benefits too; increased carbon buildup in the ocean over recent years has already caused problems with acidification, threatening coral reefs and shellfish. The alkaline output of this process, if directed where it's needed, could help redress the balance.
The team has a practical demonstration project planned for sometime in the next two years, and says there are plenty of things that still need work. For one, the researchers would love to be able to separate the gas out without a vacuum system. And mineral precipitates are fouling the electrodes on the alkalinization side, so there's plenty of progress yet to be made.
The study is open access in the journal Energy & Environmental Science.
Source: MIT