In a conventional arms race, amassing the most advanced intel and weaponry is one way to stay ahead of the enemy. However in the ongoing evolutionary battle between host cells and viruses, scientists in Spain have found that the answer to advancing in this war could lie in taking a step back in time – all the way to the early beginnings of life.
When a virus infects a cell, it hijacks the host's proteins to replicate itself. So why not deactivate these proteins to protect against infection? That's because they're needed in several cellular functions in the host and turning them off can cause harm.
In light of this, Jose Sanchez-Ruiz and his team at the University of Granada decided to try a different tack by replacing one of these proteins with an ancestral version. Their hypothesis was that since modern pathogens have co-evolved with the proteins to become what they are today, these ancient variants would be too distant on an evolutionary scale for them to co-opt. At the same time, these ancestral proteins would not have any adverse effects on the cell. In other words, they would be able to boost viral resistance without harming the host.
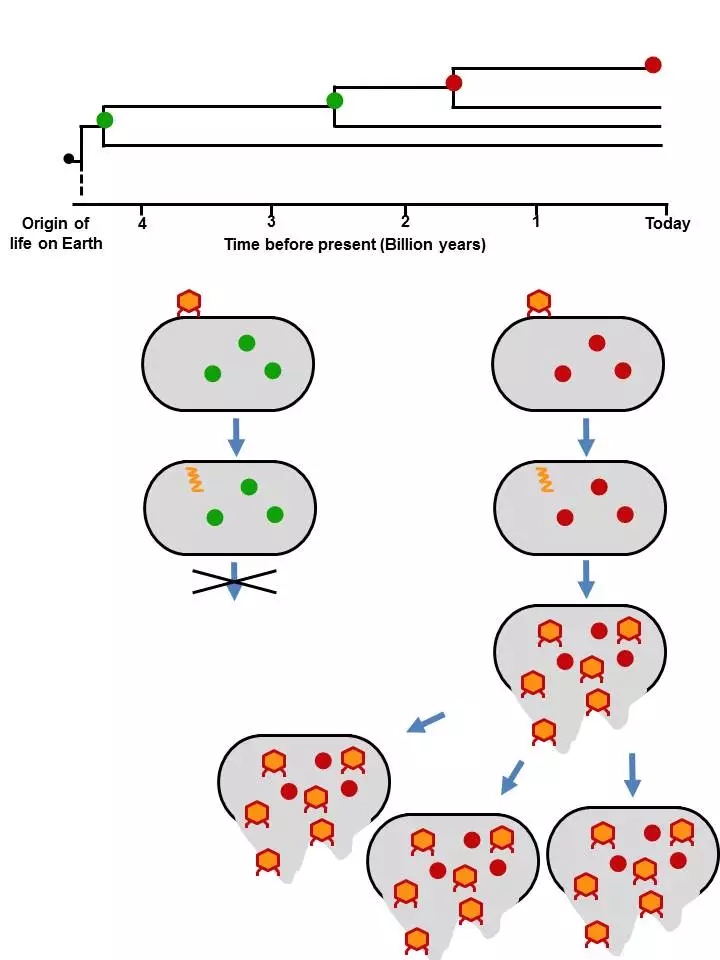
The researchers decided to focus on the protein thioredoxin, a key player in cellular reactions and one that has been around since the origin of life. More importantly, it is one of the proteins that a virus recruits in its command-and conquer-mission. Without it, the virus cannot replicate itself.
In their experiments, the researchers created seven variants of primordial thioredoxins that were plucked from four billion years of evolution and were surprised to find that they were able to function in modern E. coli bacteria, albeit with varying degrees of success. According to the study, the thioredoxin that dated back to around 2.5 billion years was the most stable.
"The modern organism is a completely different cellular environment," explains researcher Asunción Delgado, who led the experiments. "Ancestral thioredoxins had different molecular partners, different everything. The farther back we get from present, the less they work in a modern organism. But even when we get back to close to the origin of life, they still show some functionality."
More importantly, when the researchers introduced the virus T7 to the altered bacteria, they found that it was unable to hijack the primordial thioredoxin and replicate itself, thus proving their hypothesis.
Senior author Sanchez-Ruiz likens the co-evolution of pathogen and host as "an arms race," one in which the latter has been on the losing end due to its inability to evade viral hijacking. "Thioredoxin has been changing in evolution to avoid being hijacked by the virus, and the virus has been evolving to hijack the protein," he says, adding that going back in time and resurrecting ancient proteins enables scientists to "spoil all of the virus' strategy."
One field they see benefiting from this discovery is agriculture. While human-infecting viruses tend to get all the attention, crop-killing pathogens actually cause far more damage in the form of famines and mass starvation, they point out. According to the Food and Agriculture Organization, an estimated 20 to 40 percent of the world's crops are lost each year due to damage caused by pests and disease. Apart from helping boost viral resistance, the University of Granada researchers say that using ancestral variants of proteins also offers another benefit: It could help stave off crop-killing viruses without introducing dramatically different elements.
"If this is applied to plants, it wouldn't be genes from ancient bacteria … it would be the ancestral version of a gene from the same plant," says Sanchez-Ruiz. "This is genetic alteration, of course, but it is a mild genetic alteration. This is not like having a gene from one species being transferred to a different species. Also, this would not be like Jurassic Park. It would just be a comparatively small change in a gene that the plant already has."
On another note, further studies could also shed light on how evolution works on a molecular level. "What we can do is let the virus evolve to adapt to the ancestral protein and then do the experiment in reverse," says Sanchez-Ruiz. "Once it's adapted to the ancestral protein, we can test how it reacts to the modern protein. We can see if it repeats the evolution. So it would be kind of a molecular version of this Stephen J. Gould 'replaying the tape of life' idea."
The study was published in Cell Reports.
Sources: University of Granada (in Spanish); Cell Press via Phys.org




