Researchers have developed technology that creates nanoscale sacs containing a compartment within a compartment, like a Russian matryoshka doll. The novel tech is capable of delivering two drugs simultaneously or at different times.
Scientists and researchers have been hard at work developing new and better methods of drug delivery that increase effectiveness and decrease potential side effects. Nanoscale liposomes, tiny artificially created sacs comprised of an aqueous solution enclosed by a two-layer membrane of lipid (fat) molecules, are commonly used as drug carriers because they’re versatile, biodegradable, and unlikely to trigger an allergic reaction.
However, current methods of liposome synthesis limit the ability to produce complex structures and generally produce liposomes with a single drug-carrying compartment. Now, researchers from Imperial College London (ICL) have developed technology that creates liposomes with compartments within compartments, enabling far greater control over drug delivery.
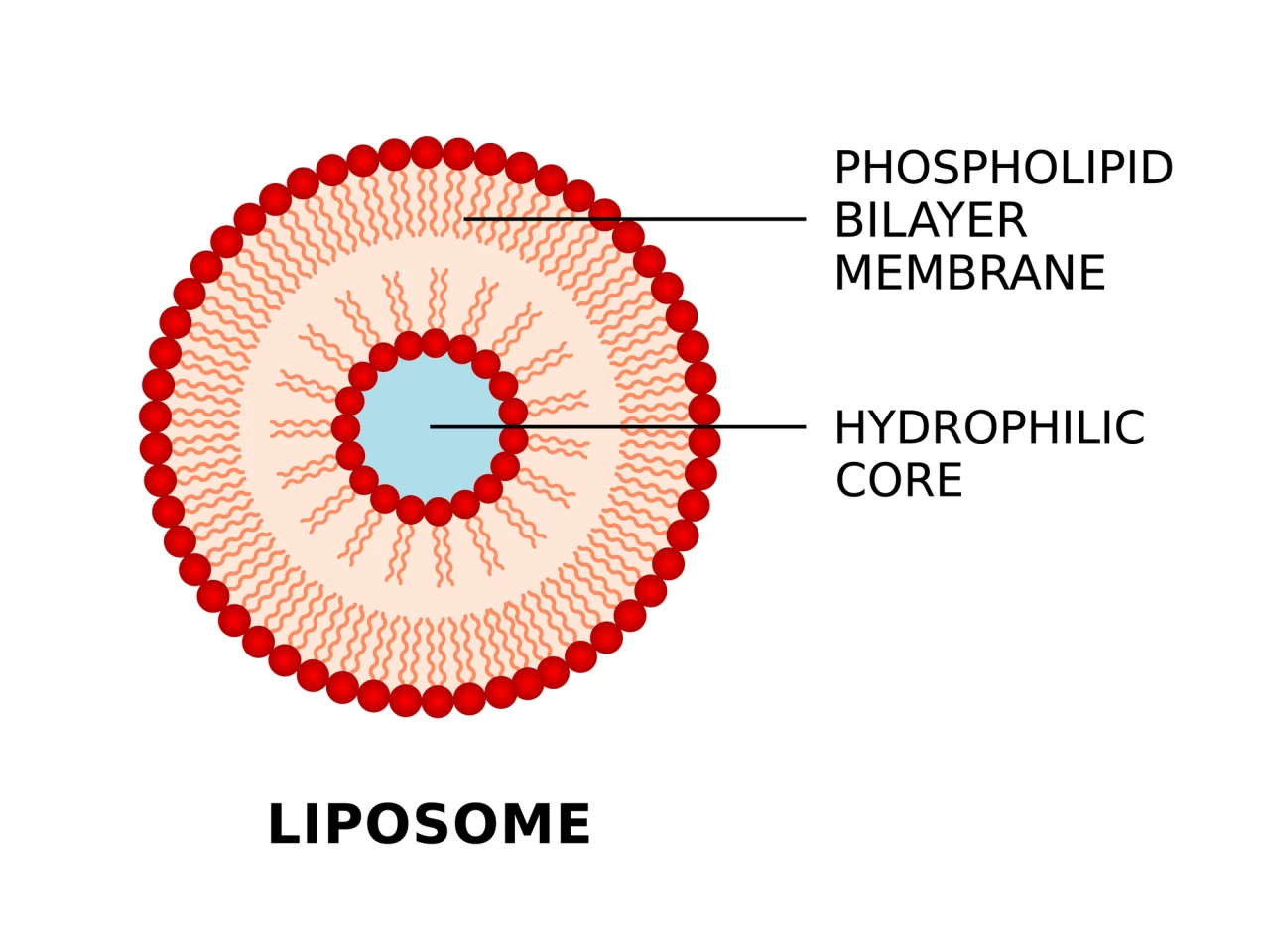
“Much like Russian dolls, our technology allows us to form particle-in-particle structures, with the ability to control all features of the particles, including the drug or vaccine encapsulated within each one,” said Dr Yuval Elani from ICL’s Department of Chemical Engineering and the study’s corresponding author. “With further research to study how these nanoparticles interact with live organisms, this advancement holds significant potential for revolutionizing both therapeutics, such as chemotherapies, and vaccines.”
Synthesizing the ‘Russian doll’ architecture of one compartment within another, which the researchers called ‘concentrisomes,’ involved layering outer lipid membranes on the inner ones. They did this by combining microfluidics, manipulating a small amount of fluids using channels in the micrometer scale, and so-called ‘click chemistry,’ a simple technique that uses readily available starting materials to produce a high yield of new compounds.
The resulting concentrisomes are microscopic: around 200 nanometers across. To give some context, human hair is approximately 80,000 to 100,000 nm wide; most proteins are about 10 nm wide, whereas a typical virus has a width of about 100 nm.

“Just as the structural complexity of animal cells makes them capable of sophisticated functions, compartmentalized nanoparticles can be tweaked to exhibit more advanced features, too,” Elani said.
The researchers could control the composition of each lipid bilayer to make the concentrisome user-defined. For example, they developed a system where one layer was responsive to temperature, that is, thermoresponsive, and the other wasn’t so that the contents were only released when a certain temperature was reached.
Through further experimentation, they demonstrated that the inner and outer concentrisome membranes could hold different drug payloads and release each at different stages. Exposing the concentrisome to a low temperature caused the outer membrane to release its payload; sequential exposure to high temperature caused release by the inner membrane. Even more impressive was the researchers’ ability to engineer the concentrisomes to synthesize new biochemicals within themselves, again triggered by a rise in temperature.
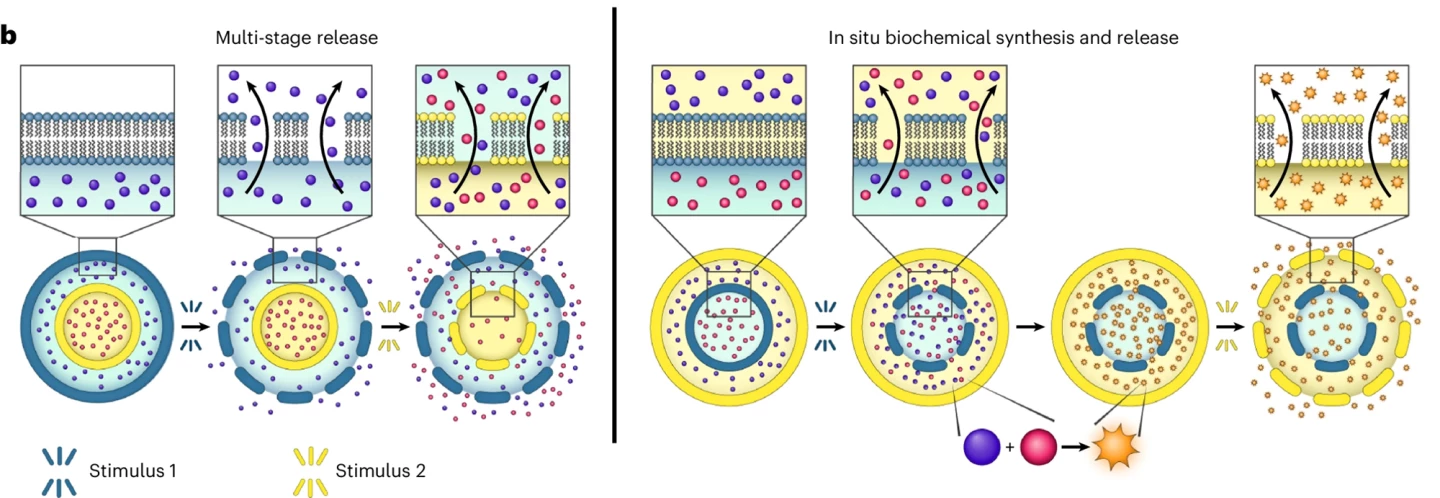
The ability to deliver two drugs simultaneously or at different time points has the potential to revolutionize combination therapies or the use of multiple drugs to treat a single disease.
The drug delivery tech is currently at the proof-of-concept stage and has not been tested in living organisms. Future research into enhancing the architectural complexity of the concentrisomes and the use of different payloads, such as genetic material, is needed before these promising preliminary findings can be translated into practical applications.
The study was published in the journal Nature Chemistry.
Source: ICL via EurekAlert!








