One dose of a new treatment, delivered by nasal spray, clears away build-ups of the toxic tau protein associated with Alzheimer’s disease from inside brain cells, improving memory, according to new research. It paves the way for new treatments for the debilitating disease.
A few years ago, abnormal clumps of tau proteins in the brain were found to be associated with Alzheimer’s disease. Since then, researchers have been working on a way of eradicating these toxic tangles, which have become a hallmark of the degenerative disease.
Now, researchers from the University of Texas Medical Branch (UTMB) have developed a breakthrough nasal spray containing antibodies that selectively target and clear away tau tangles, helping to restore cognitive function.
“This nasal spray approach opens new avenues for non-invasive delivery of tau therapeutic antibodies directly to the brain, and it holds promise for many neurogenerative diseases,” said Dr. Rakez Kayed, professor at the Department of Neurology at UTMB and the study’s corresponding author. “Our research highlights the potential of nasal tau immunotherapy to effectively target intracellular tau aggregates – a primary driver of neurodegeneration and cognitive decline in diseases like Alzheimer’s and other tauopathies.”
In healthy brains, tau proteins stabilize microtubules, which provide the scaffolding for cells and help transport nutrients. But when these proteins misfold, they can clump together into neurofibrillary tangles that disrupt the function of neurons and contribute to cognitive decline. Previous research found that disease progression occurs through a ‘seeding’ mechanism, where toxic tau seeds are released from the cell into the extracellular environment, where they propagate.
One of the major hurdles that tau-targeting treatments must overcome is targeting intracellular tau – the tau that remains inside cells and is responsible for seeding – which is something that existing immunotherapies don’t do overall. Another is crossing the blood-brain barrier (BBB), a protective barrier between the brain’s blood vessels and the cells and other components that make up the brain tissue.
The researchers’ novel therapy achieves both. Their toxic tau conformation-specific monoclonal antibody 2 (TTCM2) specifically detects and targets disease-relevant tau aggregates. Loaded into micelles, aggregates of molecules that are both water-loving (hydrophilic) and fat-loving (lipophilic), that are delivered intranasally means the treatment quickly arrives in the brain via the nose-to-brain anatomical pathway, bypassing the BBB completely.
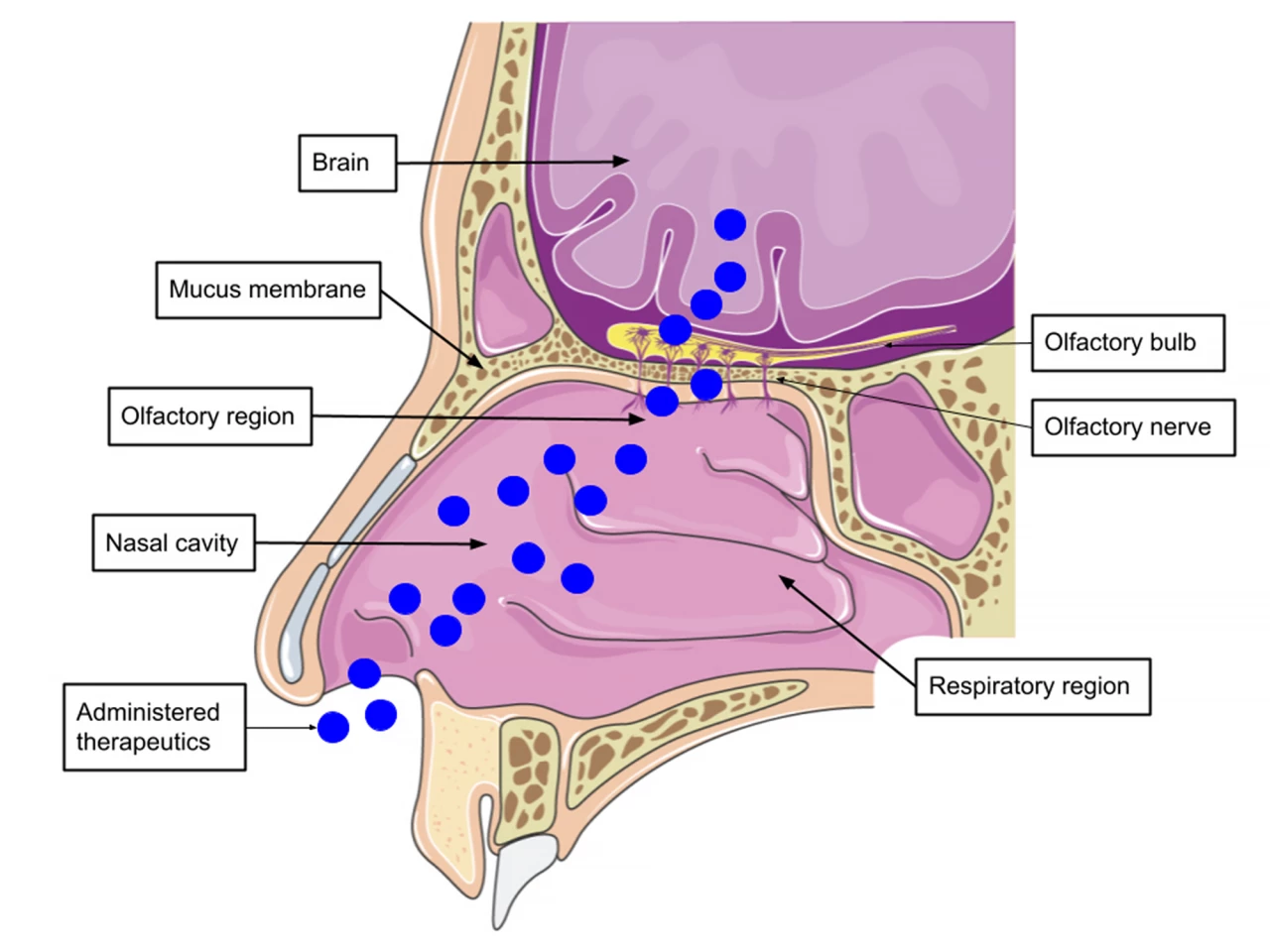
A single dose of TTCM2 was administered intranasally to aged mice that had been genetically altered to express human tau. After three hours, TTCM2 was distributed to various brain regions, including the regions’ intracellular compartments, where it cleared “seed-competent” intracellular tau aggregates and tau on the synaptic connections between neurons in the brain.
When they were subjected to behavioral testing, TTCM2-treated mice performed “markedly better” than other cohorts, suggesting that the treatment alleviated short-term memory loss in mice with advanced tau aggregates. The researchers also noticed an increase in biomarkers in the hippocampus, the region of the brain associated with memory formation and cognitive functioning. Crucial to the therapy, the researchers found, was TTCM2’s engagement with TRIM21, an intracellular (inside the cell) antibody receptor, which facilitated the clearance of antibody-bound tau aggregates.
“This method not only improves the delivery of therapeutic antibodies but also enhances their efficacy in clearing tau aggregates and improving cognitive functions,” Kayed said.
The discovery has great potential as a treatment for Alzheimer’s disease and other neurodegenerative diseases caused by a pathological buildup of tau protein.
“This advancement could significantly impact the treatment strategies for Alzheimer’s and related tauopathies, offering new hope for millions of patients suffering from these debilitating conditions,” said Sagar Gaikwad, the study’s first author and a postdoctoral fellow at UTMB.
The researchers plan to continue with preclinical trials of TTCM2 with a view to moving to human trials. Their goal is to translate these promising results into viable treatment options.
The study was published in the journal Science Translational Medicine.
Source: UTMB






