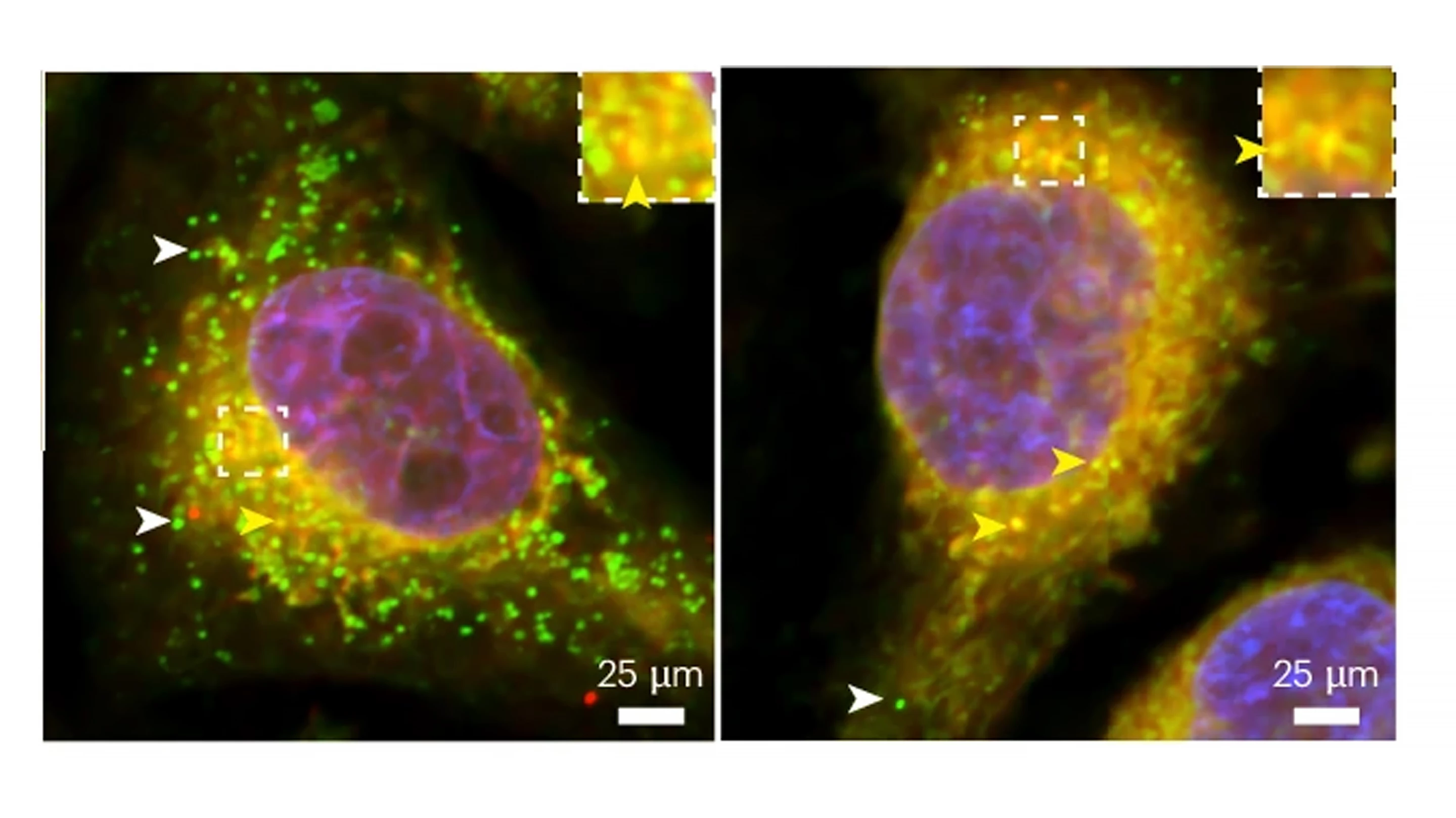
A new study has somewhat redeemed the tau protein, which up to now has been associated with the development of Alzheimer’s disease. Turns out, the protein has a ‘good guy’ role, helping to protect against harmful free radicals in the brain and promoting healthy aging.
For a while now, the tau protein has been considered a villain. Researchers have consistently found a link between Alzheimer’s (and some other neurological conditions) and toxic accumulations, called tangles, of the protein in the brain’s neurons. Consequently, there’s been a focus on developing methods of clearing away these pathological tangles.
But a new study, a collaboration between researchers from the Baylor College of Medicine (BCM) and the Duncan Neurological Research Institute (Duncan NRI) at Texas Children’s Hospital, has redeemed the tau protein by finding that it plays a ‘good guy’ role, protecting our brains from harmful reactive oxygen species (ROS) and promoting healthy aging.
“ROS are natural byproducts of various cellular functions in the body,” said Dr Lindsey Goodman, a postdoctoral fellow in the Department of Molecular and Human Genetics at BCM and the study’s lead author. “While low levels of ROS are beneficial, excess ROS is harmful to cells as it triggers the production of toxic forms of other molecules that induce oxidative stress, including peroxidated lipids.”

Brain cells require a substantial amount of oxygen to function. The metabolism of oxygen creates unstable molecules – ‘free radicals,’ of which ROS are a subset – that, in healthy amounts, support brain cell growth and cognitive functioning. They're unstable because they have an odd number of electrons. Antioxidants neutralize free radicals by donating one of their electrons, stabilizing them and reducing the damage they cause.
Oxidative stress occurs when there’s an imbalance between the production and accumulation of ROS in the cells. Long-term oxidative stress damages the brain cells and the proteins, lipids (fats), and DNA they contain, which can contribute to aging and may play a role in the development of conditions such as cancer, diabetes, and Parkinson’s disease. Lipid peroxidation is the process that causes the deterioration of the cell lipids by ROS, which at medium or high rates is toxic.
In 2015, the team at Bellen’s lab discovered that neurons exported these toxic peroxidated lipids to neighboring glial cells – cells that provide structure and nutrients to neurons and clean up the dead ones – which form them into lipid droplets that they store away during waking hours and convert into energy during sleep to maintain proper neuronal functioning.
“This process effectively removes and neutralizes these toxic lipids,” Goodman said. “In the current study, we investigated the role of tau in the formation of glial lipid droplets.”
Conducting experiments on fruit flies, Drosophila, the researchers found that glial cells require normal, naturally produced tau to form lipid droplets and to protect against neuronal ROS. They also found that glial cells obtained from rats and humans required tau for lipid droplet formation.
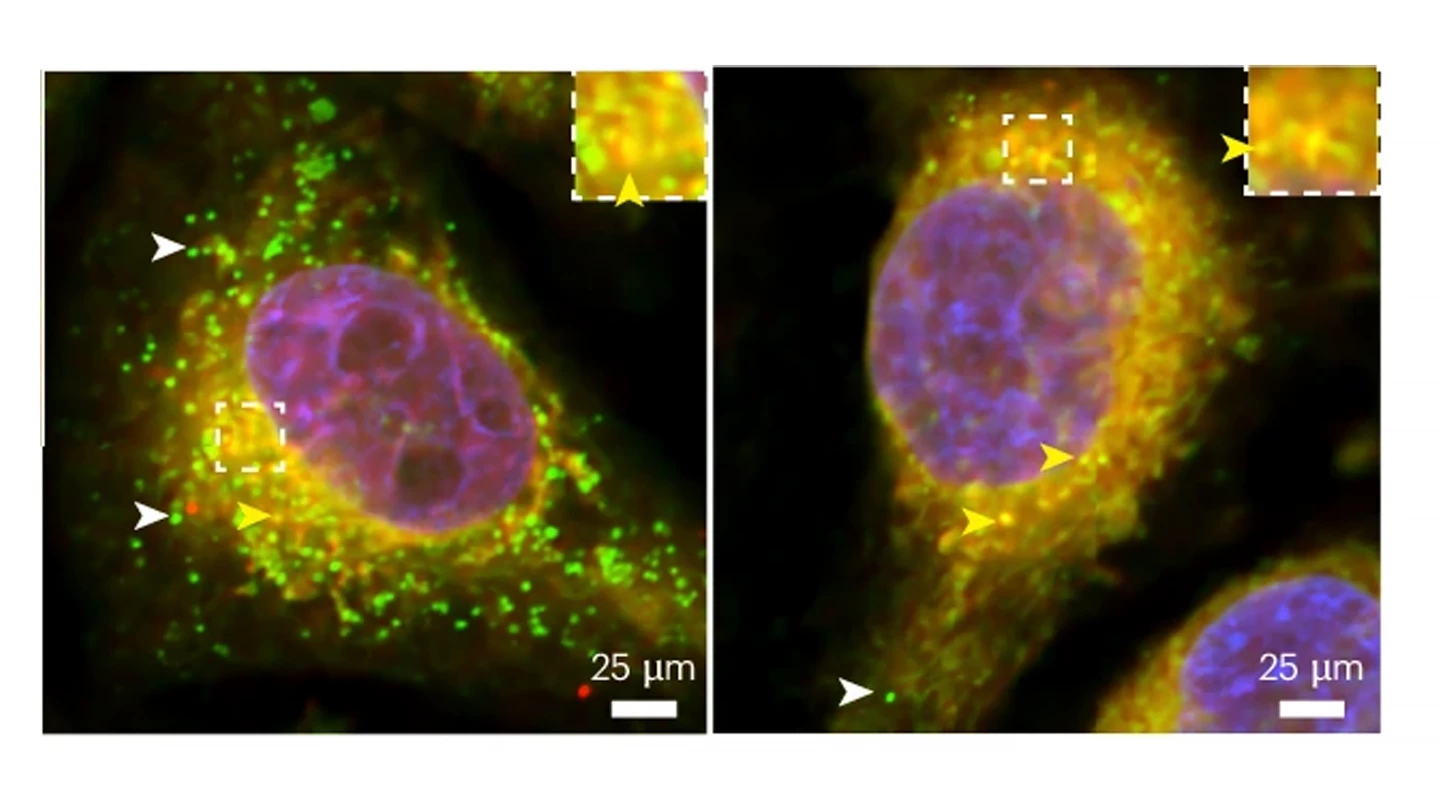
Interestingly, while the introduction of normal human tau allowed glial lipid droplets to form and mature in fruit flies lacking their own supply of the protein when the researchers introduced human tau with disease-causing mutations – which have been linked to an increased risk for Alzheimer’s disease – the glia were incapable of forming lipid droplets in response to neuronal ROS.
“This argues that mutations in tau may reduce the protein’s normal ability to prevent oxidative stress in addition to causing the protein to accumulate into the typical hallmarks of disease, as described by previous work,” said Goodman.
When the researchers used rat and fruit fly models of tau-related conditions – so-called tauopathies – that cause the disease-causing human tau protein to be over-expressed, they again observed disruptions to the formation of glial lipid droplets and the destruction of glial cells in response to neuronal ROS. It seems that, with tau, the ‘Goldilocks principle’ is at play: too little or too much of the protein is detrimental.
The researchers say that their findings pave the way for developing new ways of treating neurodegenerative conditions like Alzheimer’s.
“By revealing a surprising new neuroprotective role for tau, the study opens the door to potential new strategies to slow, reverse and treat neurodegenerative conditions,” said Dr Hugo Bellen, who leads BCM’s Molecular and Human Genetics lab and is the corresponding author of the study.
The study was published in the journal Nature Neuroscience.
Source: BCM