Coronavirus (COVID-19)
News and updates on the novel coronavirus, COVID-19.
-
Wearing face masks and maintaining social distances were a significant part of the world's reaction to the COVID-19 pandemic. Now, new research says the practices are not only effective at saving human lives, but chimp lives as well.
-
Animals that produce their own light source, through an internal chemical reaction, are a true wonder of nature – and something biotechnology scientists have been working hard to replicate and adapt for human use. They've now made a huge breakthrough.
-
Looking beyond jabs, sprays or tablets, scientists are thinking outside the box for delivering antiviral medication to prevent the spread of highly transmissible bugs. Their secret weapon? Chewing gum – but one made from a rather fascinating bean.
-
A decade-long study of 35 million Americans in 10 states has found that group A streptococcus infections have more than doubled. What's more, "strep" – which can cause a bizarre flesh-eating disease – has become increasingly resistant to antibiotics.
-
COVID antiviral Paxlovid wasn't perfect. Many patients couldn't take it due to extreme side effects but now the second generation of antivirals are on the way and they promise better efficacy and fewer drug complications.
-
How you cope in a crisis and then move on from it may have less to do with your learned life skills and instead be shaped by your base personality. A new study has found the key traits that make some people experts in 'turning lemons into lemonade.'
-
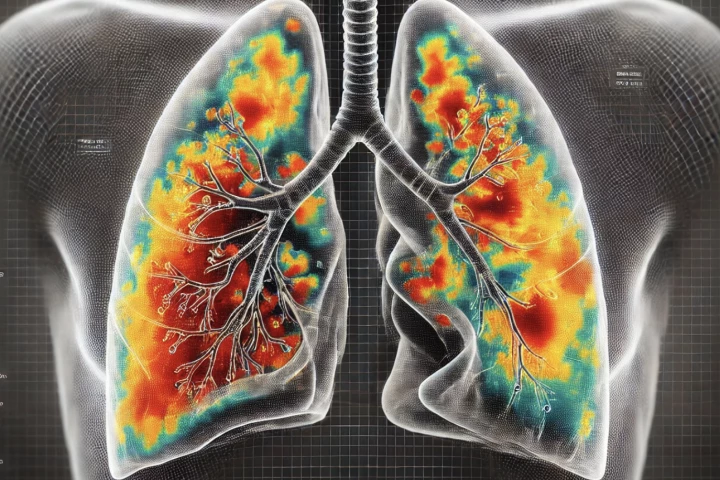
A breakthrough new AI model is able to detect the presence of different lung diseases from ultrasound videos, with 96.57% accuracy, and it is even able to distinguish whether the abnormalities are due to pneumonia, COVID-19 or other conditions.
-
The US has recorded its first death due to avian influenza. The man had been infected by a H5N1 variant with a genetic mutation, helping it target upper respiratory tract receptors – one also seen in the Canadian teen who fell critically ill last year.
-
When it comes to getting a cold or the flu, there is a commonly held belief that men take it harder than women, exaggerating their symptoms and basically acting like big ol' babies. But is this phenomenon of "man sick" a real thing? We investigate.
-
Nobody wants to get a respiratory infection, but vaccines aren't 100% effective, and constantly taking drugs can be problematic. That's where a new nasal spray may come in, as it's been shown to prevent such illnesses without the use of drugs.
-
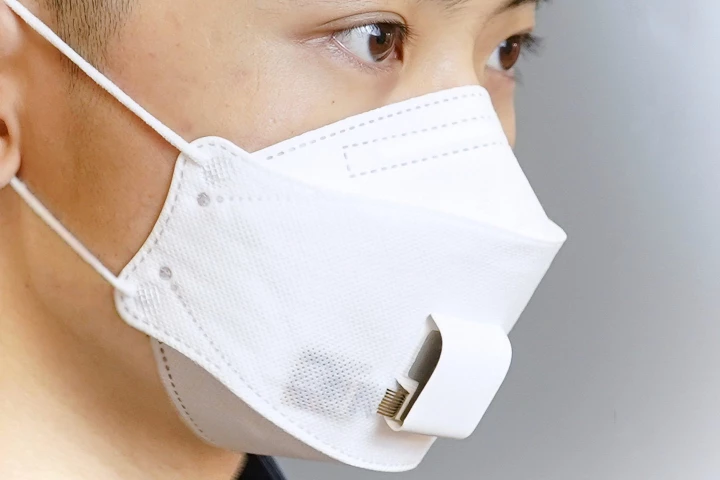
Many people still often wear masks, either to keep from spreading viruses that they've got, or to manage a respiratory problem. An experimental new mask takes things a step further, by analyzing its wearer's breath to check their state of health.
-
A new device has been shown to protect wearers from airborne viruses while leaving their face mask-free. It blocks microbes via a curtain of air which has itself been pretreated to kill any viruses present within it.
Load More