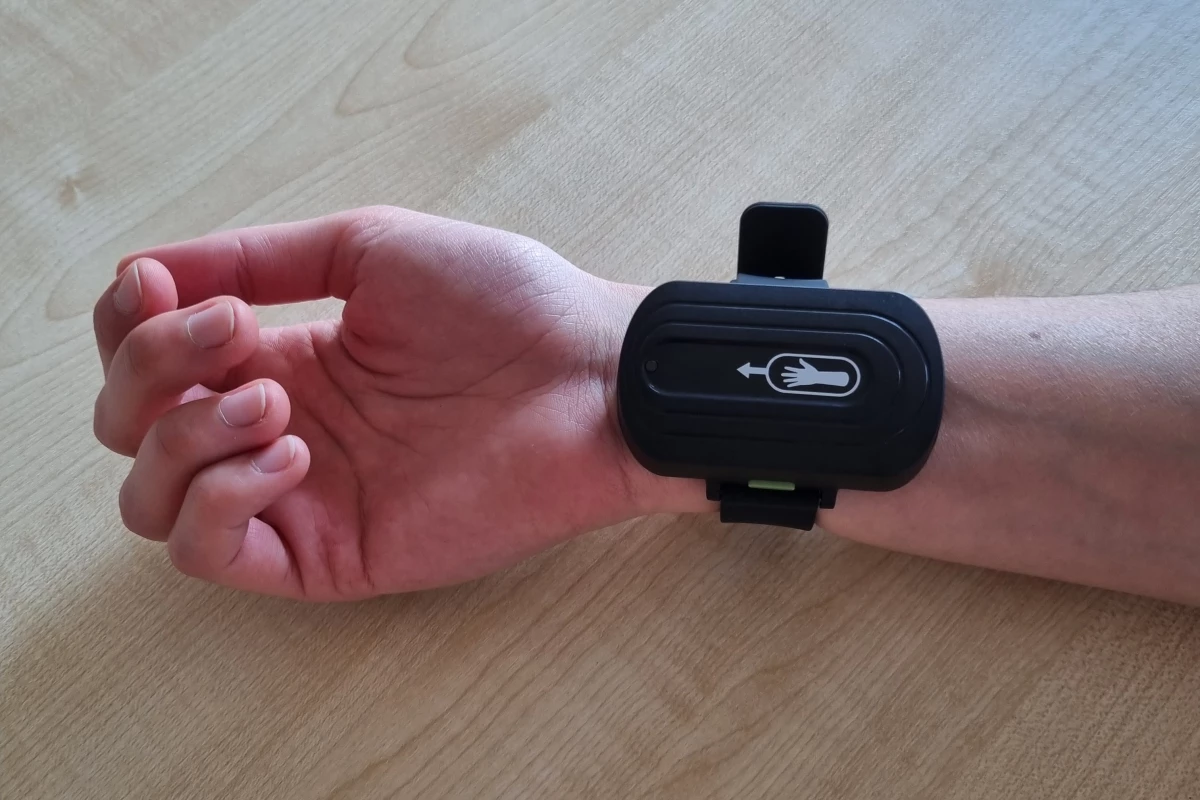
There may be new hope for people afflicted with Tourette's syndrome, in the form of a wrist-worn device. In a test of the technology, the majority of participants experienced a reduction in tic severity of at least 25%.
Tourette's syndrome causes people to experience disruptive involuntary physical and verbal tics, which typically occur multiple times per day.
Three years ago, scientists from Britain's University of Nottingham announced their finding that rhythmic electrical stimulation of the wrist's median nerve significantly reduced the frequency and intensity of those tics. More specifically, sessions of pulsed nerve stimulation produced patterns of rhythmic brain activity which were associated with tic suppression.
U Nottingham spinoff company Neurotherapuetics Ltd has now built that treatment into an app-controlled wearable device, known as the Neupulse. It was recently the subject of a double-blind clinical trial which involved 121 Tourette's patients located throughout the UK.
Half of those people were given a functional Neupulse, while the other half were given a sham device. Both groups were told to activate the wearable five days a week, at the same time each day, for 15 minutes per session. They did so over a four-week period.
It was found that 59% of test subjects using the actual device reported a reduction in tic severity at least 25% higher than anything reported by the control group. In some individual cases, the reduction was as high as 35%. It is hoped that those numbers will rise as the technology is developed further.
"The results of this trial mark an exciting step towards an effective, non-invasive treatment for Tourette syndrome that can be used at home," said the lead scientist, Neurotherapeutics CEO Dr. Barbara Morera Maiquez. "We are now focused on using the knowledge from the trial to develop a commercial device that can be made available to people with Tourette’s."
Plans call for a product to be on the market by 2026. A paper on the study was recently published in the journal MedRxive.