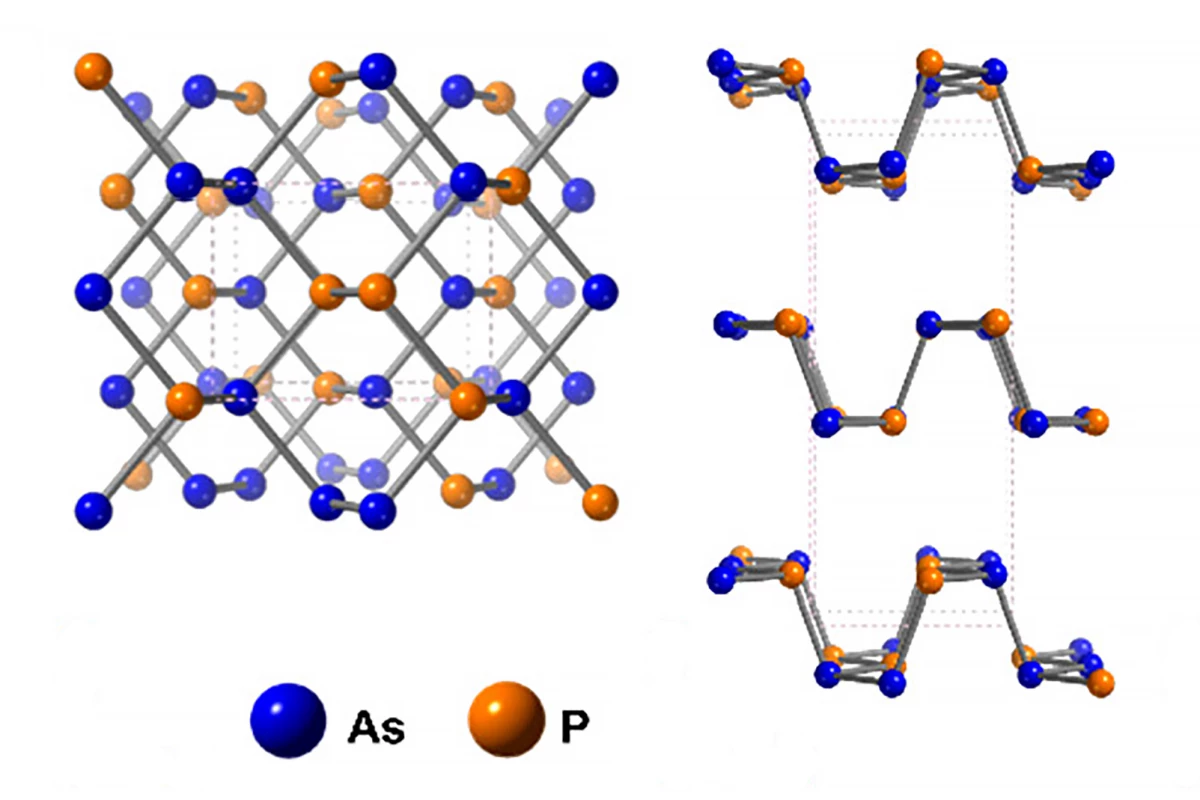
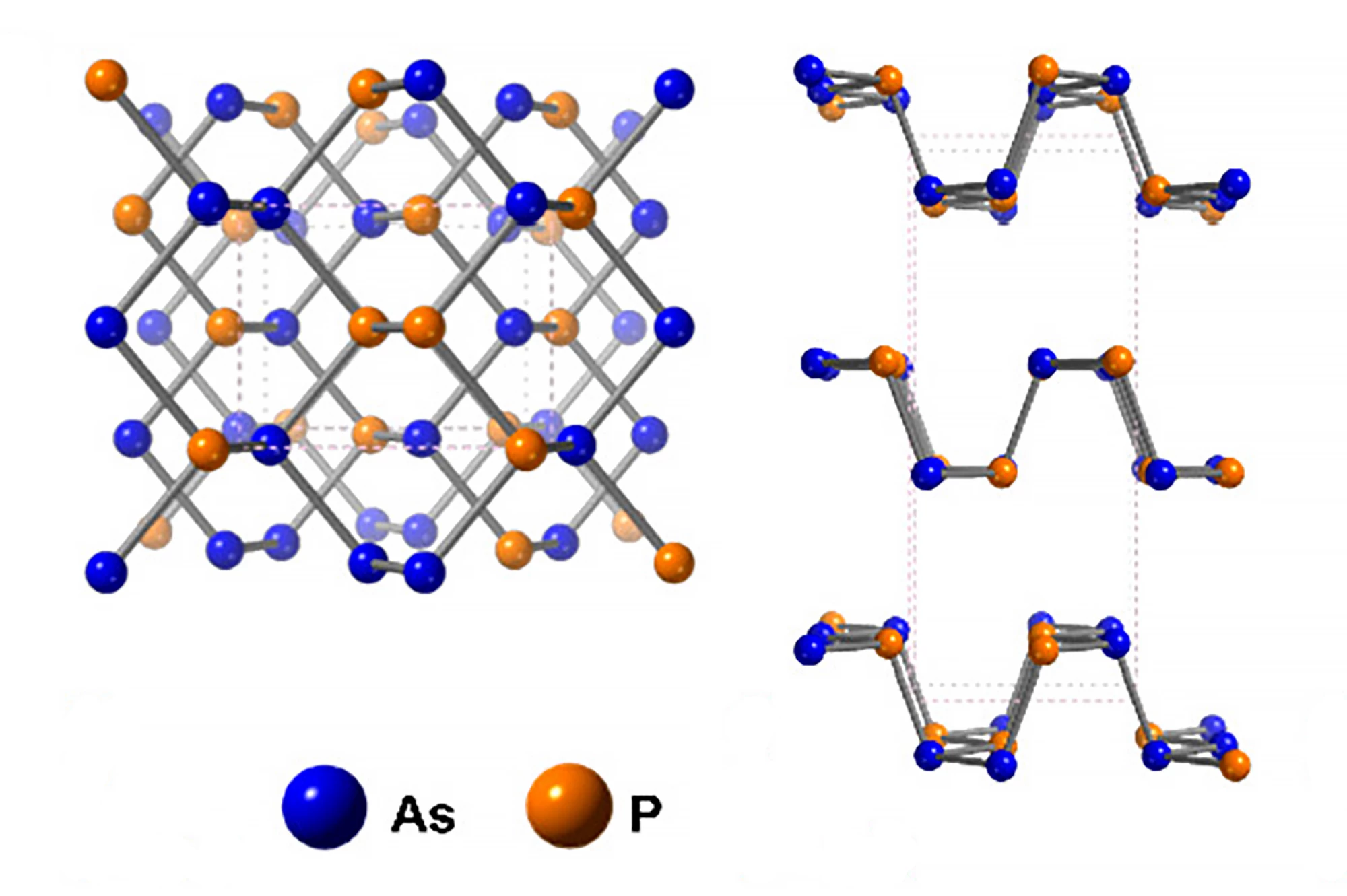
Researchers have created a new family of nanomaterials by alloying phosphorus with arsenic to create single-atom-thick ribbons that are highly conductive, making them ideal candidates for use in next-generation batteries, solar cells and quantum computers.
"Phosphorus?” I hear you ask, “but that doesn’t conduct electricity very well.” You’re right; it doesn’t, which means that, by itself, it has little to offer in practical applications and devices. However, researchers from University College London (UCL) have found that when it’s alloyed with arsenic, phosphorus becomes a whole lot more useful.
“Our latest work in alloying phosphorus nanoribbons with arsenic opens up further possibilities – in particular, improving energy storage of batteries and supercapacitors and enhancing near-infrared detectors used in medicine,” said Adam Clancy, one of the study’s corresponding authors.
By nanoribbons, the researchers are referring to one-atom-thick ribbons of phosphorus or, more correctly, phosphorene, a 2D material consisting of a single layer of artificially made layered black phosphorus, the most stable form of phosphorus. In 2019, UCL researchers discovered the potential of phosphorus nanoribbons, finding that adding them as a layer to perovskite solar cells allowed the cells to harness more energy from the Sun.
In the current study, in an effort to improve the electrical conductivity of phosphorus, they introduced a “tiny” amount of arsenic. Crystals formed from sheets of phosphorus and arsenic were mixed with lithium dissolved in liquid ammonia at -58 °F (-50 °C). The ammonia was removed after 24 hours and replaced with an organic solvent. Due to the atomic structure of the sheets, the lithium ions can only travel in one direction, not laterally, causing cracking that creates the ribbons. The researchers had created a new family of nanomaterials: arsenic-phosphorus alloy nanoribbons (AsPNRs).
They found that the AsPNRs were highly electrically conducting above 130 K (–226 °F/–140 °C) while retaining the useful properties of phosphorus-only nanoribbons. A key characteristic of the AsPNRs is their extremely high “hole mobility.” Holes are the opposite partners to electrons in electrical transport, so improving their mobility – a measure of the speed at which they travel through the material – helps electrical current move more efficiently.
Presently, to be used as an anode material in lithium-ion or sodium-ion batteries, phosphorus nanoribbons would need to be mixed with a conductive material like carbon. The researchers say that due to AsPNRs enhancing the amount of energy a battery can store and the speed at which it can be charged and discharged, the carbon filler could be dispensed with. Moreover, they say that using AsPNRs in solar cells would improve the flow of charge through the devices, enhancing the cells’ efficiency.
“The arsenic-phosphorus ribbons have also turned out to be magnetic, which we believe comes from atoms along the edge, which makes them potentially of interest for quantum computers too,” Clancy said. “More widely, the study shows that alloying is a powerful tool for controlling the properties and thus applications and potential of this growing nanomaterial family.”
The researchers say their AsPNRs could be produced at scale in a liquid that could then be used to apply them, in volume and at low cost, for use in different applications.
The study was published in the Journal of the American Chemical Society.
Source: University College London