Engineers at ETH Zurich have developed a new, surprisingly simple method for merging two metals into one nanocrystal structure. The team says that this could allow almost any two metals to be combined, creating brand new types of “intermetallic nanocrystals” that could be useful for a whole range of applications.
Mixing metals into alloys has long been useful for fine-tuning specific properties, like conductivity and strength, depending on what they’re needed for. Potential applications only get wider if alloys can be made into nanocrystals, tiny balls of atoms arranged in a repeating pattern. The problem is, there’s no standard way to make these intermetallic nanocrystals, and as such only a few combinations out of potentially tens of thousands have been made so far.
For the new study, the ETH Zurich engineers found a new way to make intermetallic nanocrystals that, they say, is so intuitive that they were surprised no one had tried it before. It’s based on amalgamation, where one solid metal is dissolved into a second metal that’s in a liquid form – usually one with a low melting point like mercury.
In this study, the team started with nanocrystals of one metal, added molecular compounds called amides containing the second metal, then heated the mixture to 300 °C (572 °F). This causes the chemical bonds in the amide to break up, and that second metal gathers as a liquid on the surface of the nanocrystals. Soon it seeps in, forming a new crystal lattice where the atoms of the two different metals become regularly dispersed. Once it cools down, an intermetallic nanocrystal is waiting.

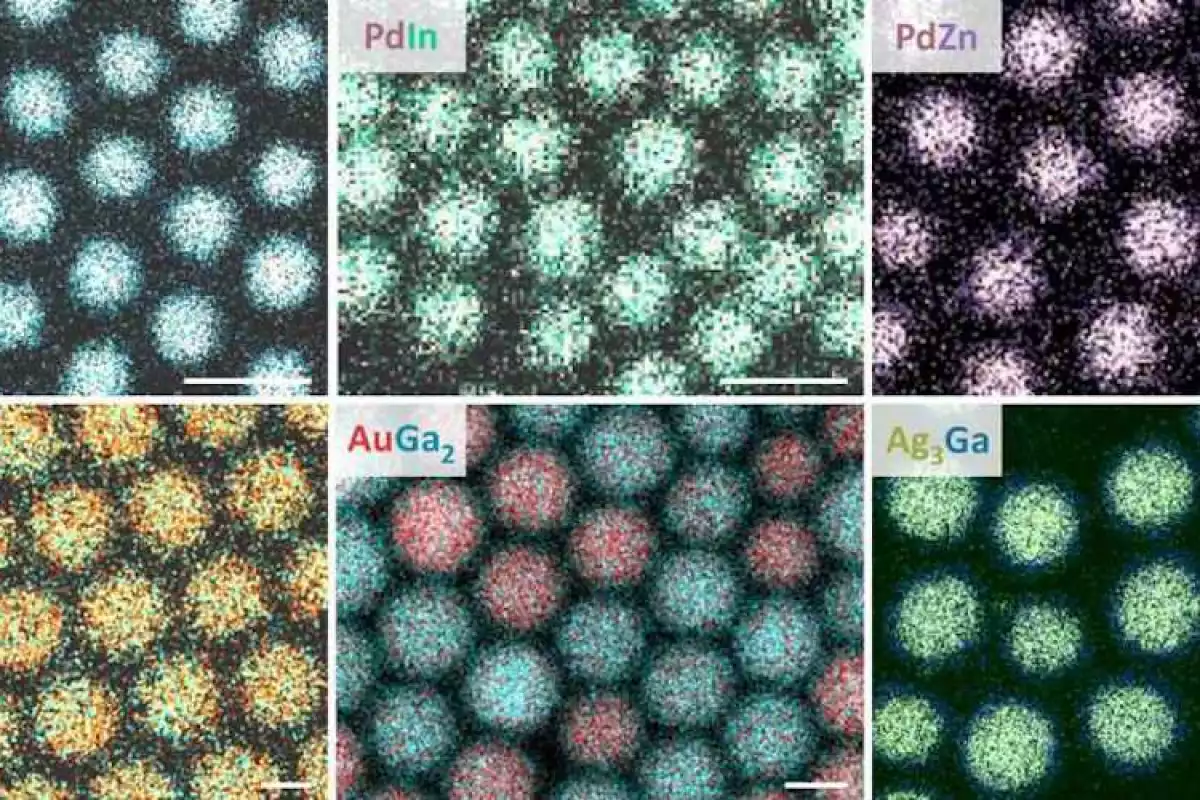
Bottom: electron microscope images of this process at work.
“We are amazed how efficient the amalgamation is at the nanoscale,” says Maksym Yarema, lead author of the study. “Having one liquid metal component is the key to fast and uniform alloying within each nanocrystal.”
Using this method, the team managed to make intermetallic nanocrystals mixing several different pairs of metals. Gallium was popular thanks to its low melting point of 30 °C (86 °F), and in this study it was coupled up with gold, silver, copper, nickel, and palladium. The lattermost also worked well as a base, with the team mixing it with indium and zinc.
Importantly, the team says that almost any metals could be used in this technique, mixed in at different ratios, and forming different-sized nanocrystals. All up, that opens a huge range of applications as catalysts, in batteries, data storage devices and other electronic components.
The research was published in the journal Science Advances.
Source: ETH Zurich