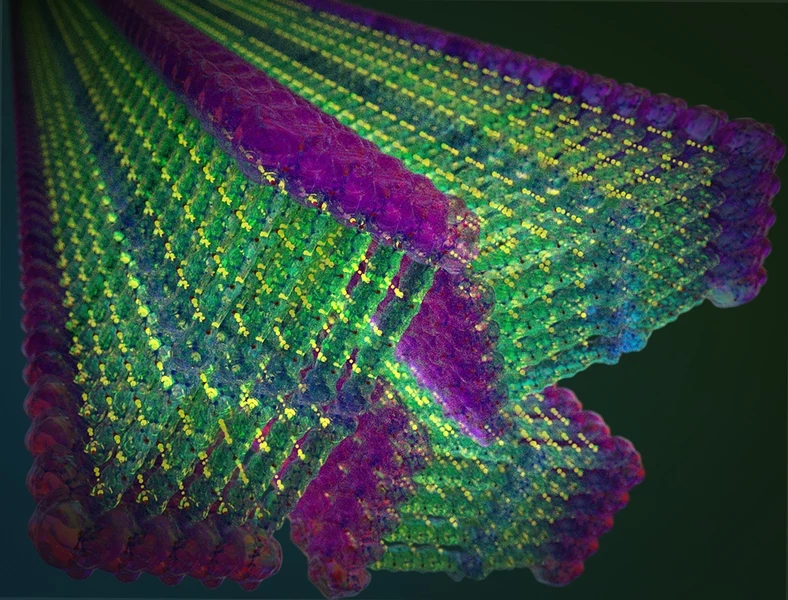
By taking some inspiration from nature and some from the way the synthetic fiber Kevlar is formed, scientists at MIT have developed self-assembling nanoribbons they say are stronger than steel. With their unique set of properties and means of fabrication, the scientists say these novel materials could find all kinds of uses, ranging from water filtration to powering electronic devices.
The self-forming nanoribbons are inspired in part by the way molecules can assemble themselves into membranes or other important structures in nature. Recreating this with engineered molecules is anything but straightforward, however, with scientists finding some success by having them assemble themselves in water, though that is where they have previously run into trouble.
“These small-molecule-based materials tend to degrade rather quickly,” explains Julia Ortony, assistant professor in MIT's Department of Materials Science and Engineering. “And they’re chemically unstable, too. The whole structure falls apart when you remove the water, particularly when any kind of external force is applied.”
Over the course of several years, Ortony and her team have been tinkering away with a new class of small molecules they hoped could address these shortcomings, and have now landed on a solution. These molecules feature an outer section that is hydrophilic and likes to interact with water, an inner section that is hydrophobic and doesn’t like interacting with water, and strong Kevlar-inspired hydrogen bonds in the middle that enables them to join tightly with other molecules.
The team came up with this recipe after trial and error involving dozens of molecule designs, but found this one most suitable for their means. That’s because the delicate mix of hydrophobic and hydrophilic segments, along with dense network of hydrogen bonds, causes the individual molecules to move around in water in just the right way, as some parts are attracted to the liquid while others are repulsed, though all cling to each other quite strongly.
When water is added the molecules assemble themselves into long ribbons just a nanometer thick, which were found to be stronger than steel. These were then stretched into long threads that could be dried out and handled, with the team finding they could hold 200 times their own weight. The material also boasts an incredibly high surface area, at 200 square meters for gram of material.
"This high surface-to-mass ratio offers promise for miniaturizing technologies by performing more chemistry with less material,” explains Ty Christoff-Tempesta, first author of the study.
One area where these types of materials with a high surface area have lots of potential is in water purification. The MIT team explored these possibilities by coating the ribbons in certain molecules and used them to pull heavy metals such as lead and arsenic from contaminated water. The scientists are also investigating how the ribbons can form part of advanced electronic devices and batteries, though they note it is still early days for the research.
“We were excited to see that our modifications to the molecular structure were indeed amplified by the collective behavior of molecules, creating nanostructures with extremely robust mechanical properties,” says Ortony. “The next step, figuring out the most important applications, will be exciting.”
The research was published in the journal Nature Nanotechnology.
Source: MIT