Just like standard lithium-ion batteries, sodium-ion batteries work by sending ions back and forth between a pair of electrodes in a liquid electrolyte, but current designs have their limitations. A research team in Russia has discovered a new bright, blue mineral they say could solve one of the problems with these experimental devices, which shape as a far more cost-effective form of energy storage than today's solutions.
Owing to the cheap and widely abundant nature of salt, sodium-ion batteries have emerged as a cost-effective alternative to the lithium-ion chemistry that powers today’s laptops, electric vehicles and smartphones. While we are seeing some exciting advances that promise to bring sodium-ion batteries up to speed, there are a still a few operational problems to solve before we see them perform as reliably as their tried and trusted lithium-ion counterparts.
One of these relates to the cathode, which as one of the battery’s two electrodes plays a key role in the travel of ions back and forth and the generation of power. The trouble with current versions of sodium-ion batteries is that as they are cycled, inactive sodium crystals tend to build up on the cathode’s surface, which soon proves fatal to the device.
Scientists at Russia’s St Petersburg University may have found a more free-flowing solution, tucked away in lava flows from the Tolbachik Volcano, which erupted in 1975-1976 and again in 2012-2013. The team has discovered dozens of unique minerals here in the past few years, but describe their latest finding as unusual.
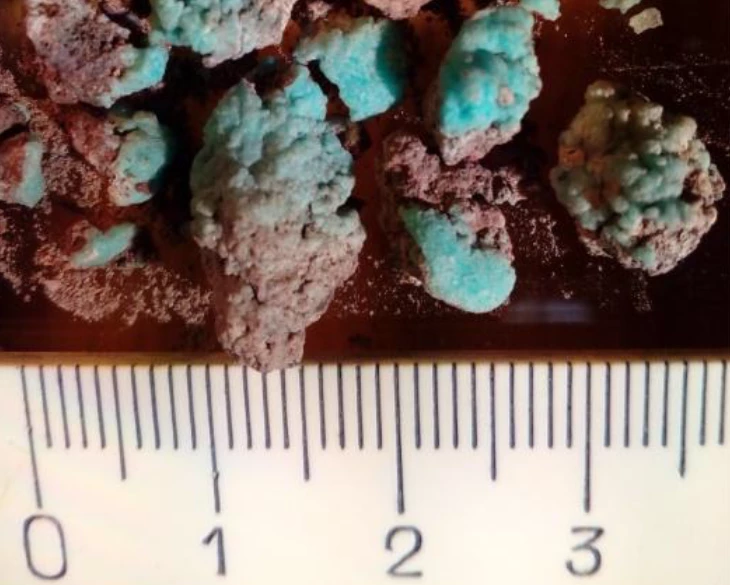
Dubbed “petrovite,” the new mineral is a bright blue aggregation of sodium sulphur, copper and a “very rare” coordination of oxygen atoms, seen only in a couple of other compounds. These make up a porous framework consisting of voids and channels that allow sodium atoms to move freely throughout, according to the scientists, who say this makes petrovite a promising structure for ionic conductivity and cathode material in sodium ion batteries.
“At present, the biggest problem for this use is the small amount of a transition metal – copper – in the crystal structure of the mineral,” says Stanislav Filatov, Professor at St Petersburg University. “It might be solved by synthesizing a compound with the same structure as petrovite in the laboratory.”
There’s obviously a lot of work to be carried out before this discovery translates to a proof-of-concept sodium-ion battery, and even more before it presents a solution to our energy storage woes. But at the very least, the scientists have uncovered a pretty new mineral to look at in the meantime.
The research was published in the journal Mineralogical Magazine.
Source: St Petersburg University



