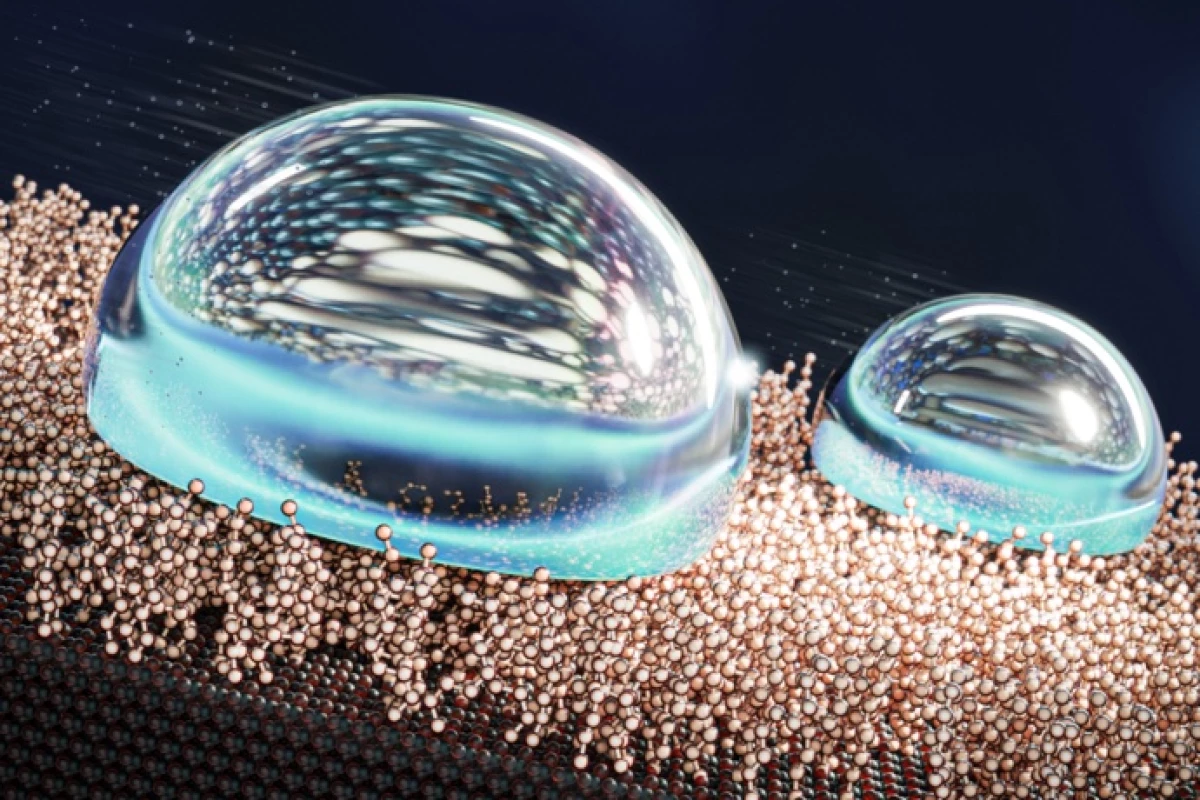
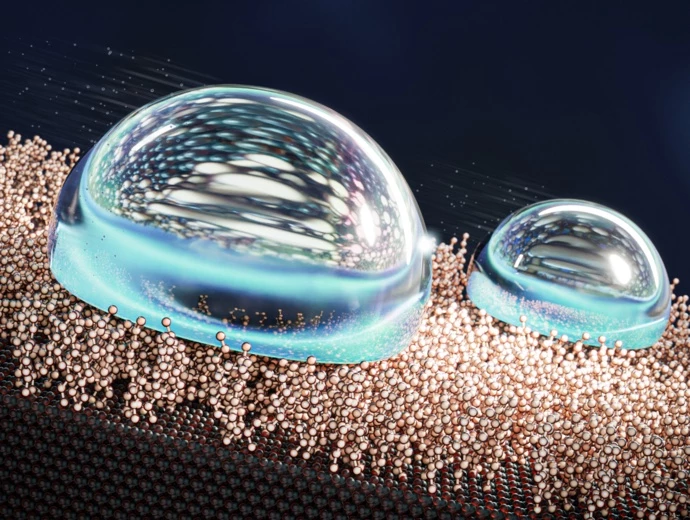
Scientists have developed what they call the most water-repellent surface ever. By giving it a liquid-like coating that defies usual designs, water will roll off the surface at angles 500 times shallower than other superhydrophobic materials.
The ability to repel water is important for many materials, particularly in the automotive, marine and aerospace industries. Many superhydrophobic surfaces work by trapping a layer of air or liquid, which causes any water that lands on it to ball up into droplets and roll off more easily. But an emerging technology creates what are called liquid-like surfaces (LLS), which have layers of highly mobile molecules that act like liquids but are tethered to substrates so they don’t escape. The end result is like a lubricated surface that water slides right off.
In the new study, scientists at Aalto University in Finland developed a new LLS out of molecules called self-assembled monolayers (SAMs) coating a silicon substrate. By tuning conditions like the temperature and water content in the reactor during production, the team could control how much of the silicon the SAMs covered.
When the SAMs covered much of the surface, it became superhydrophobic, causing water to form droplets and roll off. That in itself was to be expected – but to the surprise of the researchers, low SAM coverage also made for a slippery surface. And it did so without the water beading, which has long been thought to be necessary for superhydrophobicity.
“It was counterintuitive that even low coverage yielded exceptional slipperiness,’ said Sakari Lepikko, lead author of the study. “We found that, instead, water flows freely between the molecules of the SAM at low SAM coverage, sliding off the surface. And when the SAM coverage is high, the water stays on top of the SAM and slides off just as easily. It’s only in between these two states that water adheres to the SAMs and sticks to the surface.”
The team says that some versions of their SAM surfaces are the most water-repelling materials ever reported – superhydrophobic surfaces usually boast sliding angles (the angle at which water will roll off) as low as 5°. But the Aalto team reports that theirs can be an astonishing 0.01°, meaning water will run off basically any surface that’s not perfectly level.
The more common measure of hydrophobicity is what’s called the contact angle, which is given by how sharp a curve water droplets form on the surface. But it’s hard to apply that measure here when the SAM surfaces allow water to spread into a film but still roll off easily.
As intriguing as the SAM coating is, the researchers do acknowledge that it’s still fairly thin and will disperse easily. But they plan to continue working to improve it, so that it could eventually help in a range of industrial use cases.
“Things like heat transfer in pipes, de-icing and anti-fogging are potential uses,” said Lepikko. “It will also help with microfluidics, where tiny droplets need to be moved around smoothly, and with creating self-cleaning surfaces. Our counterintuitive mechanism is a new way to increase droplet mobility anywhere it’s needed.”
The research was published in the journal Nature Chemistry.
Source: Aalto University