After a decade in the works, researchers at University of California, Los Angeles, have successfully devised a way to produce cement with 98% less CO2 emissions than traditional methods.
The UCLA team achieved this by decomposing limestone – the key raw material involved in making cement – to access calcium oxide, aka lime, without releasing carbon dioxide in the process.
The team is calling this method ZeroCAL, short for zero carbon lime. It's a big deal because there's a massive opportunity to reduce global greenhouse gas emissions. Traditionally, heating limestone in a kiln to produce lime results in 1 lb of CO2 emitted for every lb of cement produced.
How does ZeroCAL work?
ZeroCAL skips the aforementioned fossil-fuel powered process of heating limestone. Instead, it starts with dissolving the sedimentary rock feedstock in a water-based solution containing a common industrial acid. Next, calcium is separated via membrane nanofiltration. Finally, an electrochemical process is carried out to produce calcium hydroxide – a zero-carbon precursor for cement and lime production.
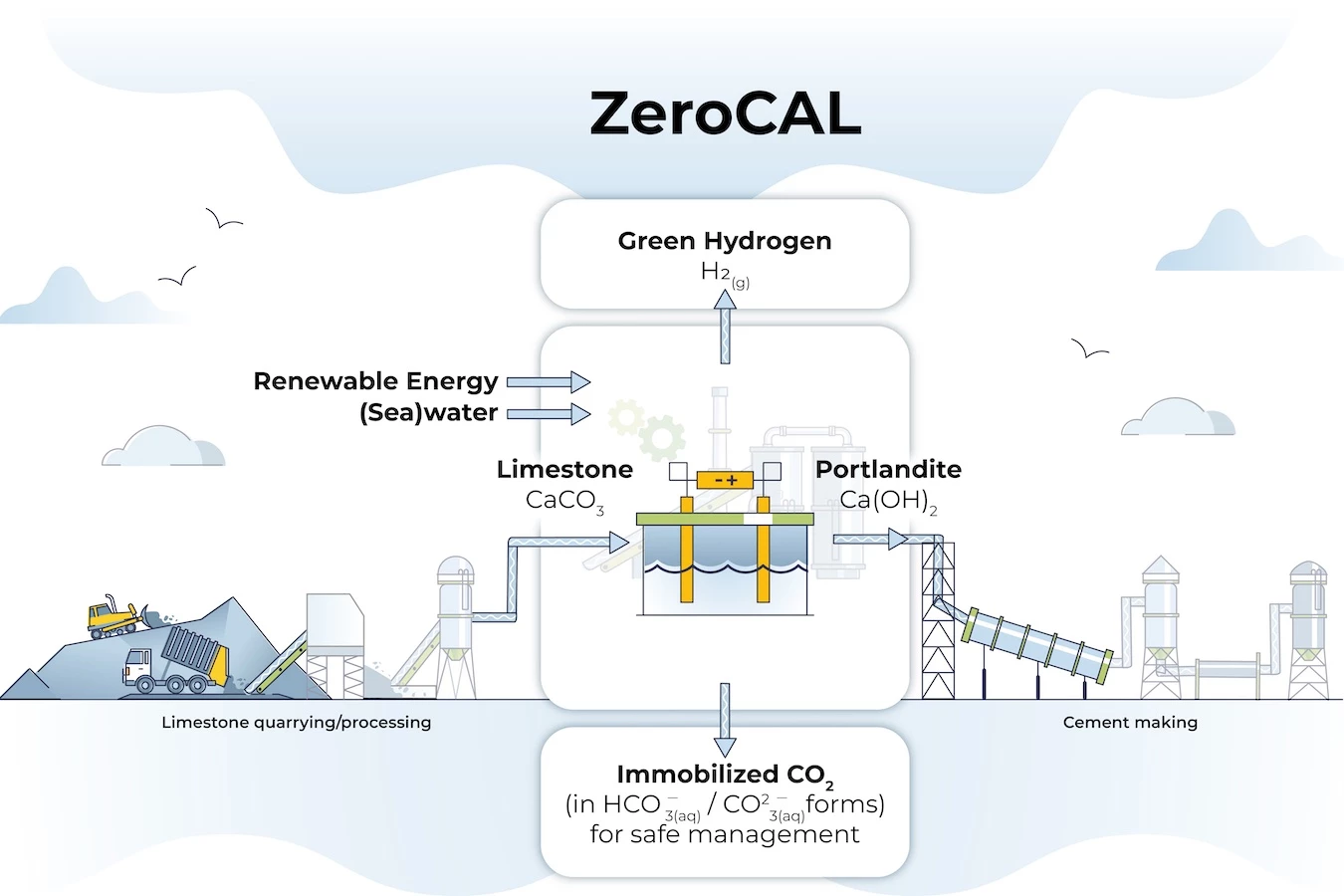
That goes beyond just reducing the emissions from processing limestone to just 1.5%. "It addresses the carbon emissions resulting from limestone’s decomposition while providing clean hydrogen and oxygen to heat the cement kiln," notes team lead Gaurav Sant. "Second, it enables onsite decarbonization while making use of existing kilns and limestone feedstocks without having to build separate carbon-capture and storage facilities.”
A greener path forward for cement production
While ZeroCAL is indeed impressive, the process isn't yet perfect. It uses more energy than traditional methods, and still uses a lot of water. That said, research into simplifying unit operations and better utilization of the electrolytically produced acid and base co-products is underway. That could eventually help ZeroCAL achieve energy-use parity.
The research team is collaborating with India's largest cement manufacturer, Ultratech Cement, to build a demonstration plant that will produce several metric tons of lime per day using the ZeroCAL process.
Cement production accounts for 8% of CO2 emissions worldwide. Hopefully we'll start seeing this process and others like it go mainstream in the near future and make a dent in that figure.
The main challenges to this endeavor are cost and energy input. Low-carbon cement is simply more expensive to produce, and still requires vast quantities of power and water. Plus, margins on regular cement are already thin. So we'll need to see more innovation in this space before green options become the norm around the world.
A paper on the project has been published in the American Chemical Society’s journal Sustainable Chemistry and Engineering.
Apart from ZeroCAL, there are a couple of other notable projects we've seen that rethink cement. In May, we learned about Cambridge University researchers chucking used concrete into steel-processing furnaces to produce “reactivated cement” as a byproduct while purifying iron. The process could make for completely carbon-zero cement when using renewable energy.
There's also Sublime Systems, which presented a zero-carbon cement solution using a novel electrochemical process last year. It landed a $75 million investment from global cement giants Holcim and CRH just last month. The MIT spinout is set to open a commercial plant in the US with a capacity of 30,000 tons per year in 2026.





