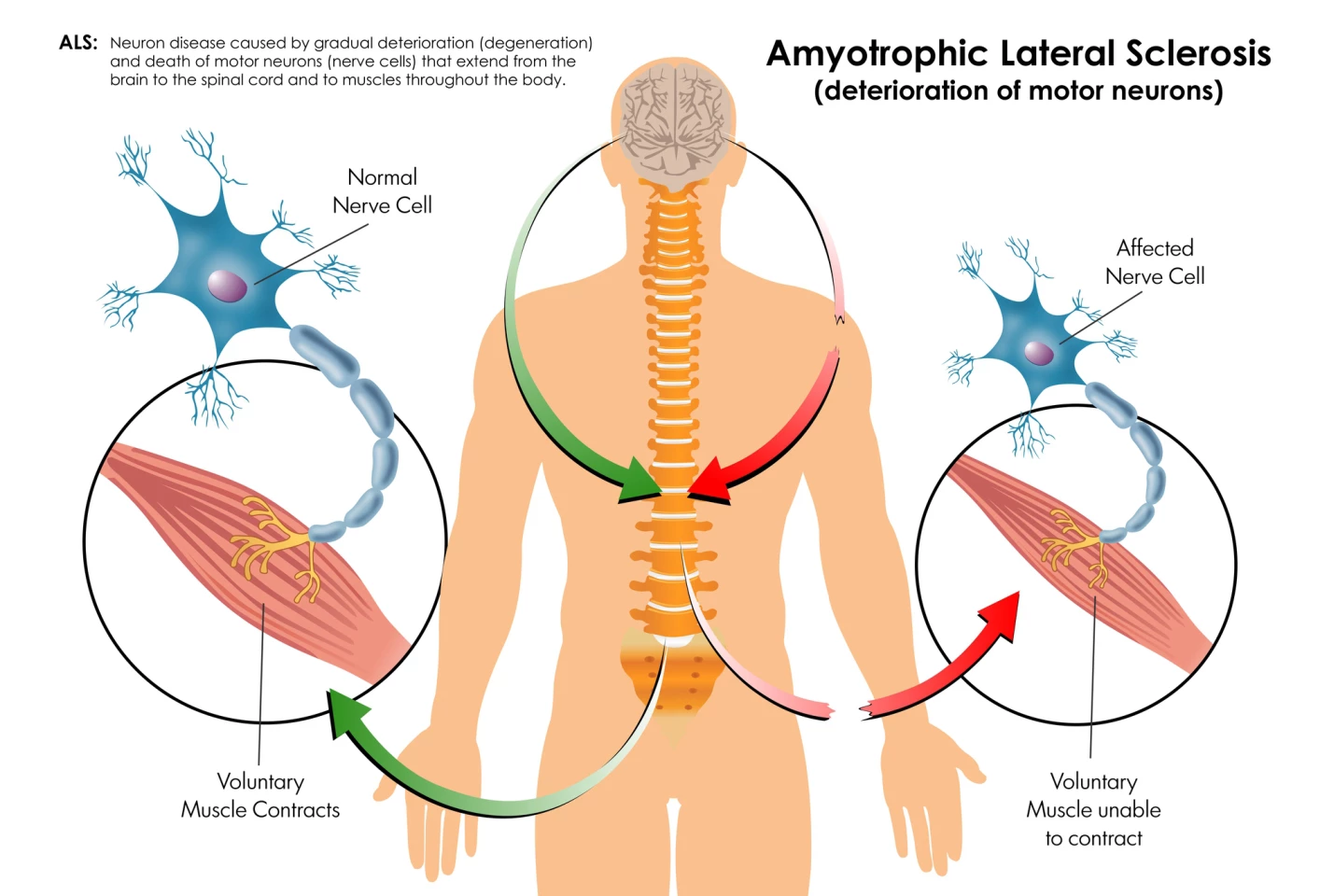
Amyotrophic lateral sclerosis (ALS), or Lou Gehrig's disease, affects nerve cells in the brain and spinal cord, called motor neurons, that control voluntary muscle movements like walking, talking, and breathing. As the neurons die and can’t send messages to the muscles, loss of muscle control worsens over time and is eventually fatal.
The US Food and Drug Administration (FDA) has approved several drugs that help manage symptoms or slow the disease’s progress, but there’s no current treatment that reverses the progression of ALS. In short, there’s no cure. That’s where Spinogenix, Inc. comes in.
Spinogenix, a clinical-stage biopharmaceutical company, has developed SPG302, a unique once-a-day pill that regenerates the gaps, called synapses, between neurons to restore communication. Following promising results from clinical trials to evaluate the drug’s safety, the FDA has approved the company’s Investigational New Drug (IND) application, paving the way for further trials.
“Having completed the Phase 1 safety study in healthy subjects in Australia, we are thrilled to have gained FDA acceptance of our US IND for SPG302 in ALS,” said Stella Sarraf, Spinogenix’s CEO and founder. “SPG302’s unique approach to regenerating synapses offers a fundamentally different treatment modality, focusing on synapse loss, which is central to ALS. Current treatments have not sufficiently met the needs of ALS patients, as slowing disease progression alone is not enough. We are committed to advancing SPG302 with the hope of providing a new, transformative therapeutic that can significantly improve the lives of those battling this devastating disease.”
US Federal law requires a drug to be the subject of an approved marketing application before being transported across state lines. If a drug manufacturer wants to undertake clinical testing in many states, it must apply to the FDA for an exemption to that law. The IND is how they do it.
SPG302’s early-stage clinical trials in Australia with healthy adults demonstrated that it’s well-tolerated and produces therapeutic levels that match the results seen in preclinical animal models. Spinogenix started dosing ALS patients in April 2024 and has received significant interest from people wanting to enroll in the trial.
If SPG302 is proven effective in this next round of clinical trials, it will be a watershed moment in ALS treatment.
“ALS is a complex and varied disease, affecting cognitive and motor functions as well as speech and respiration,” said Dr. Merit Cudkowicz, Chair of the Massachusetts General Hospital Department of Neurology, Professor of Neurology at Harvard Medical School, and member of Spinogenix’s Advisory Board. “Spinogenix’s new approach works at the synaptic level to regenerate synapses. This first study in people with ALS is an important step towards determining whether SPG302 helps recover lost functions in motor and cognitive symptom domains.”
We’re sure that, like us, many people will be watching this space.
Source: Spinogenix






