FDA
-
In a comprehensive new study looking at 39,763 different foods and drinks from the biggest 25 companies in the country, scientists discovered that almost 20% rely on synthetic food dyes to attract consumers. Now, the fight is on to ban them for good.
-
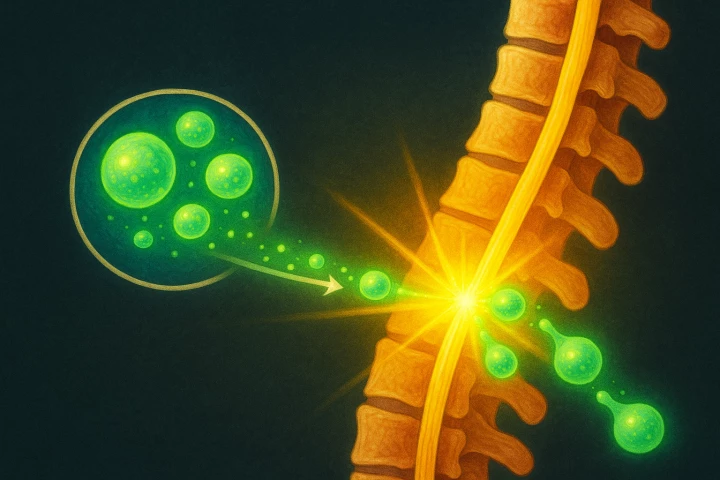
A paradigm shift in the way we treat spinal injuries is now in sight, with the world's first regenerative cell therapy approved for a Phase I clinical trial. It's a historical milestone that could reverse what has, until now, been an incurable injury.
-
A new antibiotic to relieve stubborn urinary tract infections and a blood-clot dissolving treatment for acute ischemic stroke will be commercially available in the coming months. It's been nearly three decades since adjacent drugs have hit the market.
-
Americans will soon have access to an infusion treatment that provides round-the-clock relief of Parkinson's symptoms. The US FDA has green-lit this innovative drug delivery system, which is expected to be available in the fourth quarter of 2025.
-
HIV has become a more manageable condition in recent years, but a full cure remains elusive. Now, scientists have found promise in permanently eliminating the virus, thanks to a drug already approved by the FDA to fight cancer.
-
The US Food and Drug Administration has approved the use of a novel painkiller for short-term moderate-to-severe pain in adults. It’s the first of a new class of analgesics to be approved in over 20 years - and, importantly, isn't addictive.
-
Take control of your food and water safety with the first-of-its-kind EcoTracker, a user-friendly pocket-sized device that almost instantly assesses contaminants in fruits, vegetables and meats, as well as the quality of your drinking water.
-
After the FDA proposed pulling some cold and flu medications from shelves in the US, an Australian law firm has launched a class action against Johnson & Johnson, claiming that it has knowingly marketed and sold ineffective decongestants for years.
-
In a much-needed update to 2003 data on attention-deficit/hyperactivity disorder in the US, a new report has found that 15.5 million American adults have been diagnosed with the condition – and many have been let down by poor access to treatment.
-
A pioneering once-a-day pill that regenerates nerve cell connections damaged by ALS has been FDA-approved for ongoing clinical trials. The drug is now being given to those with ALS and could be a watershed moment in the treatment of the fatal disease.
-
A drug used to treat asthma has been shown to substantially reduce the risk of potentially life-threatening reactions in people aged one and older with multiple common food allergies, including peanuts, following accidental exposure.
-
The FDA has granted clearance to the first AI-powered medical device to assist physicians in detecting all 3 common skin cancers. Providing a more accurate way of identifying skin cancer will enable patients to access necessary treatment more quickly.
Load More