Much research into Alzheimer's focuses on the buildup of brain plaques as a primary cause, but the case is far from closed, particularly in the eyes of a research team at New York University. In newly published research, the scientists detail how declining acidity in cellular cleaning organelles called lysosomes acts as even earlier evidence of the disease's onset, and they've shown how restoring proper acid levels could save neurons from irreversible damage.
The notion that the build-up of amyloid beta plaques in the brain causes the neurodegeneration seen in Alzheimer's has been around for decades, and guided much of the research into potential treatments. However, a number of promising drugs developed to target these plaques have failed in recent years. While the amyloid hypothesis is still a very active arm of Alzheimer's research, some are looking elsewhere for answers, and those studying the functions of lysosomes are uncovering some highly valuable insights.
Lysosomes are tiny sacs found in the cells of many animals, and are packed with acidic enzymes that break down, remove and recycle waste products. They are known as the cell's garbage disposal system for this reason, and recent research has begun to show how the dysfunction of this system could play a role in the neuronal damage seen in Alzheimer's.
One interesting 2019 study showed that amyloid proteins are able to flip their molecular structures so that lysosomes are unable to recognize them and clear them away. This resulting buildup of failed lysosomes is thought to contribute to neuronal damage, and the research backed up a 2015 Yale study showing that this can simultaneously enhance the buildup of toxic proteins.
This new study adds further weight to this line of thinking, and does so by demonstrating how lysosome dysfunction can drive neuronal damage well before the amyloid plaques are fully formed. The authors used mice bred to develop Alzheimer's and tracked acid levels inside the lysosomes as the cells became injured due to the disease.
Imaging of the lysosomes showed that as neurons took on damage their acidity levels decreased. Some of these lysosomes became enlarged as they interacted with vesicles packed with waste that had failed to be broken down, causing the vesicles to gather in flower-like bulges around the cells' membrane. Some of these vesicles were found to contain early forms of amyloid beta, which went on to form filaments inside the cell and, in some of the damaged neurons, develop into fully-formed plaques.
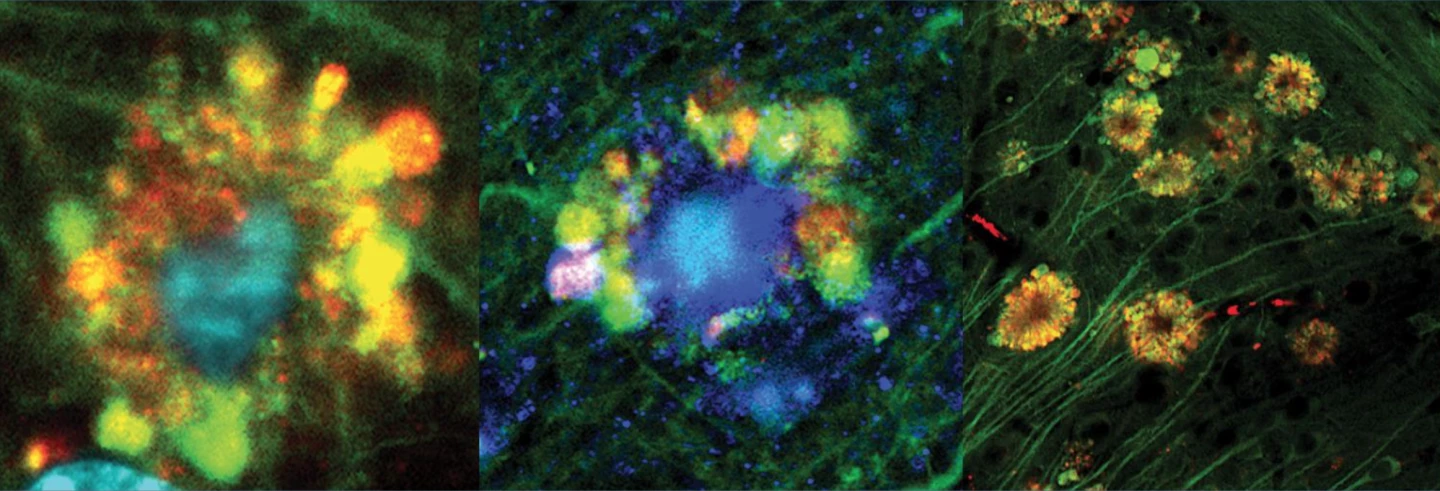
“Our results for the first time sources neuronal damage observed in Alzheimer’s disease to problems inside brain cells’ lysosomes where amyloid beta first appears,” says study lead investigator Ju-Hyun Lee, PhD. “Previously, the working hypothesis mostly attributed the damage observed in Alzheimer’s disease to what came after amyloid buildup outside of brain cells, not before and from within neurons."
The scientists are now working to develop therapies that target this form of lysosome dysfunction. In previous work, they had tied lysosome failure to a gene long-associated with Alzheimer's disease called PSEN1, and they've now been able to demonstrate how this form of neuronal damage can be reversed by restoring acidity in the lysosomes to normal levels.
“This new evidence changes our fundamental understanding of how Alzheimer’s disease progresses; it also explains why so many experimental therapies designed to remove amyloid plaques have failed to stop disease progression because the brain cells are already crippled before the plaques fully form outside the cell,” says study senior investigator Ralph A. Nixon. “Our research suggests that future treatments should focus on reversing the lysosomal dysfunction and rebalancing acid levels inside the brain’s neurons."
The research was published in the journal Nature Neuroscience.
Source: New York University





