Currently, small metal implants either have to be surgically removed from the body once they're no longer needed, or just left inside indefinitely – both of which can lead to complications. Now, however, scientists have devised a method of breaking them down in place using liquid metal.
Led by Asst. Prof. Giovanni Traverso and postdoctoral researcher Vivian Feig, a team at MIT drew upon a process known as liquid metal embrittlement.
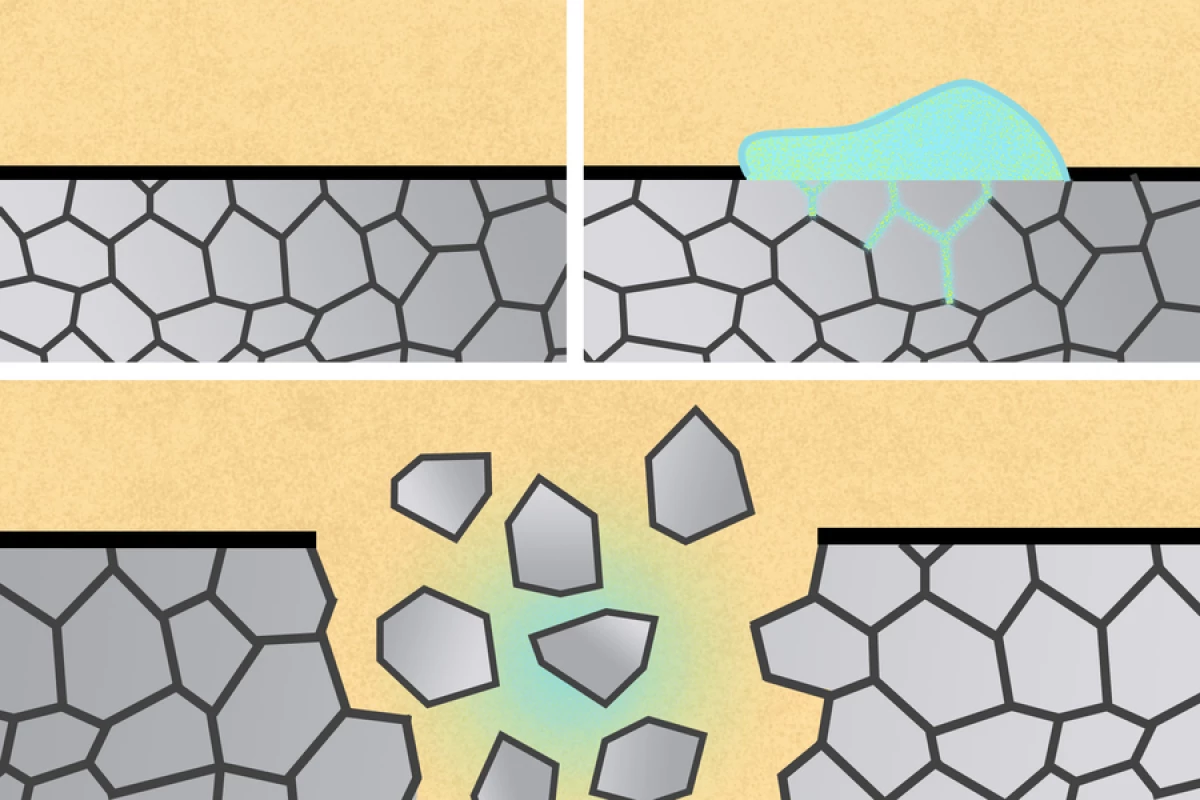
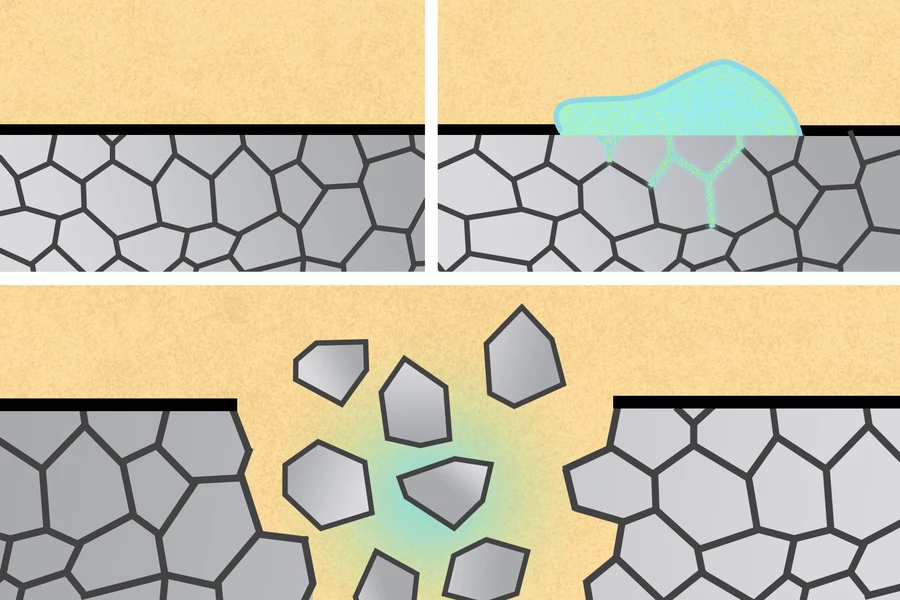
In this phenomenon, hard metals such as zinc or stainless steel disintegrate upon contact with certain types of liquid metal. This happens as the liquid metal penetrates the solid metal's grain boundaries, which are the borders between the tiny crystals it's composed of.
Initially, the scientists were looking at harnessing the process in order to break down devices implanted in the gastrointestinal (GI) tract. They knew that a soft metal called gallium works well on hard aluminum, so they experimented with a gallium alloy – eutectic gallium-indium (EGaIn) – along with a partially aluminum drug-delivery device.
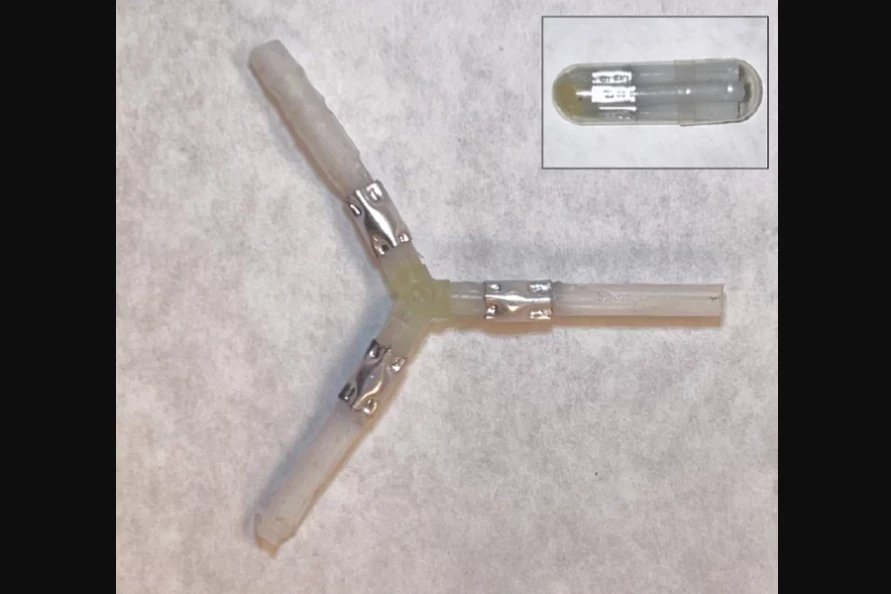
That Y-shaped prototype device consisted of medication-filled polymer arms joined to a polymer hub by aluminum connector tubes. The idea was that once it got inserted into a patient's GI tract, it would harmlessly lodge in place and gradually release its drug payload, until it disintegrated and was passed with the feces.
In animal studies, after the device had been put in place in the GI tract, a solution containing EGaIn was orally administered. As the liquid passed over the device, it caused the aluminum connectors to break down, allowing the device to fall apart and be passed. Importantly, rodent studies indicated that EGaIn is non-toxic and biocompatible, although further research has to be conducted on its effect on humans.
Following their success with the drug-delivery device, the scientists were also able to break down an aluminum stent implanted in esophageal tissue. Moving beyond the GI tract, they then tried painting EGaIn onto aluminum surgical staples of the type used to hold wounds closed – the removal of regular surgical staples, by traditional means, can sometimes actually damage the healed tissue.
It was found that the liquid metal caused the aluminum staples to disintegrate in a matter of minutes. Additionally, if the staples were used in a real-world scenario, the resulting aluminum fragments reportedly wouldn't be a problem.
"For staples, our design is such that the tissue is held together because of a bridge supporting two counterposing legs; if the bridge is dissolved, the legs of the staple can be easily removed," Feig told us. "Alternatively, if there are fragments left inside the tissue, we have observed that they can be easily rinsed out."
The research is described in a paper that was recently published in the journal Advanced Materials.
Source: MIT