Studies have already shown that electrical stimulation helps heal wounds, including broken bones. Scientists have now developed what could be a better way of delivering electricity to such bones, in the form of a biodegradable implant.
In order to electrically stimulate broken bones, externally powered electrodes would currently have to be surgically implanted at the wound site. Once the bone had healed, they would then need to be surgically removed.
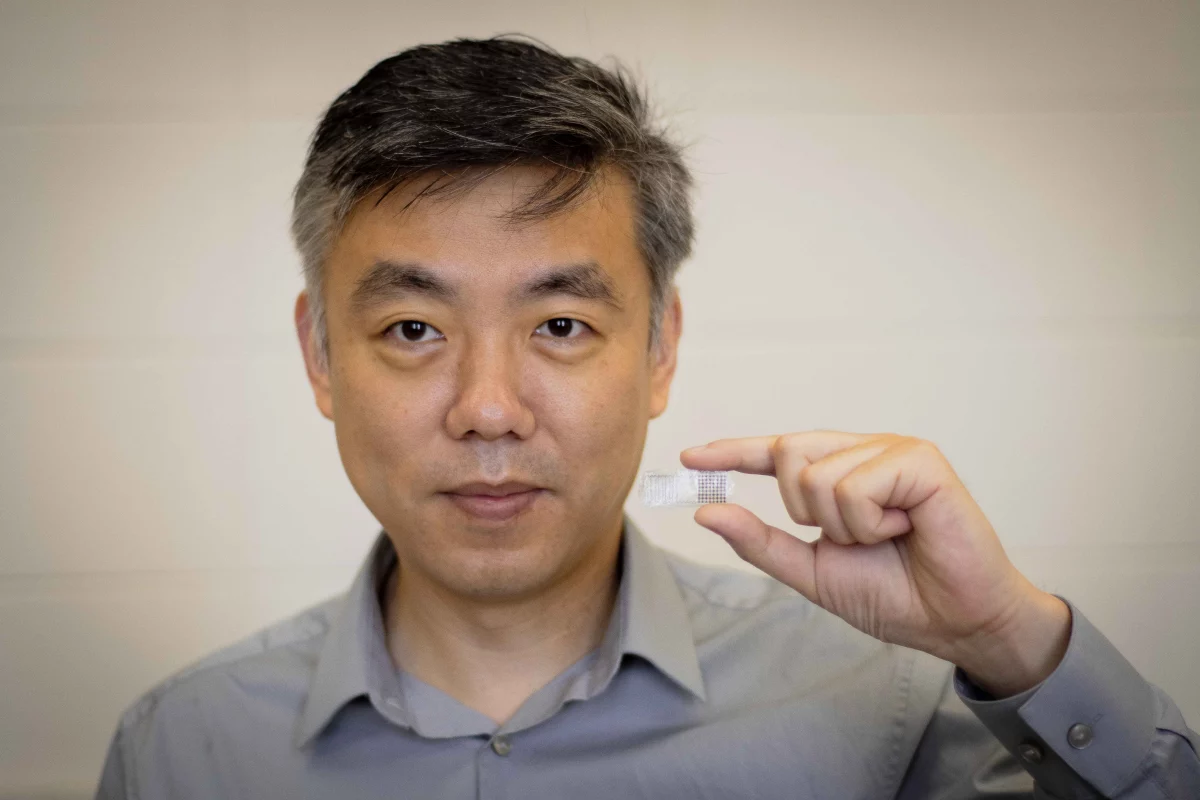
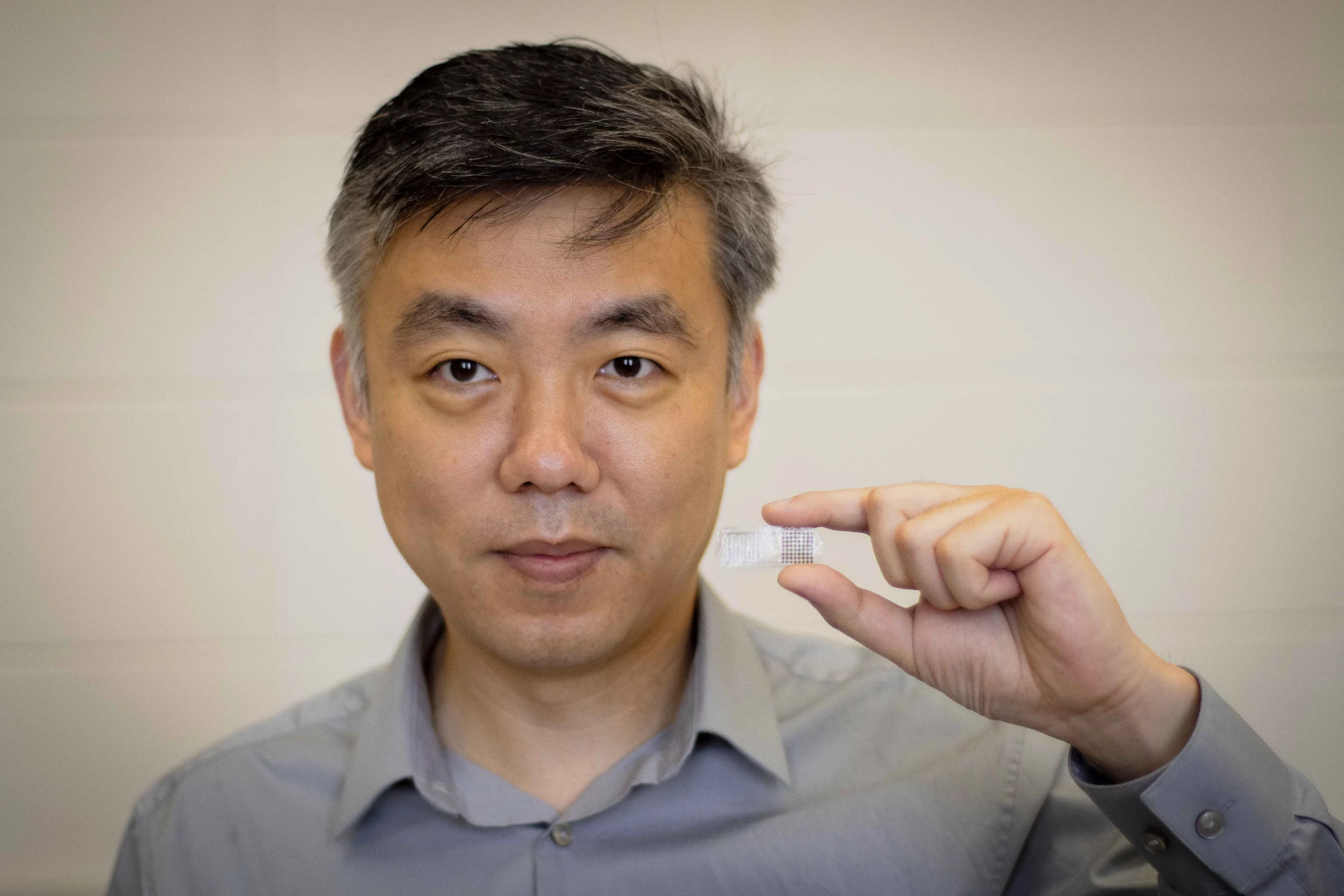
Seeking a simpler, less invasive alternative, a team led by the University of Wisconsin-Madison's Prof. Xudong Wang created a self-powered electrical patch that is surgically placed onto a bone-break site, but that is harmlessly absorbed by the body once its job is done. It's called the fracture electrostimulation device, or FED.
The base of the device consists of an FDA-approved biocompatible polymer, on top of which is a thin-film triboelectric nanogenerator that's hooked up to two electrodes. That nanogenerator converts mechanical energy produced by body movements into an electrical current, much in the same way that rubbing your sock feet against the carpet produces a static charge. The electrodes deliver the current to the bone.

In lab tests, the device allowed rats to recover from a fractured tibia within six weeks, which was significantly quicker than the recovery time for an untreated control group. That said, the rats remained relatively active during the six-week period, so the nanogenerator was consistently able to produce about 4 volts. By contrast, humans are usually advised to immobilize limbs with broken bones.
"We may need the device to respond to other types of internal mechanical sources, like blood pressure changes," says Wang. "It will be very interesting and impactful to address the development from animal to human."
The research is described in a paper that was recently published in the journal Proceedings of the National Academy of Sciences.
Scientists at the University of Connecticut are developing a technology that is similar, but also a bit different. It's a biodegradable piezoelectric material which is implanted at a bone fracture site, then proceeds to generate an electrical field when triggered by externally applied pulses of ultrasound.
Source: University of Wisconsin-Madison