A new review has highlighted how skin tone can affect the safety and effectiveness of some medications and why the current way we run clinical drug trials needs to change to include more historically underrepresented populations.
Clinical trials are essential for evaluating the effectiveness and safety of medications in human subjects. While the overarching goal of biomedical research is to improve the health and well-being of an entire population, participants in clinical trials tend to be far from diverse. An analysis of 32,000 individuals who participated in new drug trials in the US during 2020 found that only 8% were Black, 6% Asian, 11% Hispanic, and 30% were aged 65 and older.
A new review by Simon Groen, an assistant professor of evolutionary systems biology in the Institute of Integrative Genome Biology at the University of California, Riverside, and Sophie Zaaijer, a consultant and researcher affiliated with UC Riverside who specializes in diversity, equity, and inclusion (DEI) in clinical trials, investigated how one aspect of race – skin tone – affects how medications work.
“Our review paper concludes that melanin, the pigment responsible for skin color, shows a surprising affinity for certain drug compounds,” said Groen. “Melanin’s implications for drug safety and dosing have been largely overlooked, raising alarming questions about the efficacy of standard dosing since people vary a lot in skin tones.”
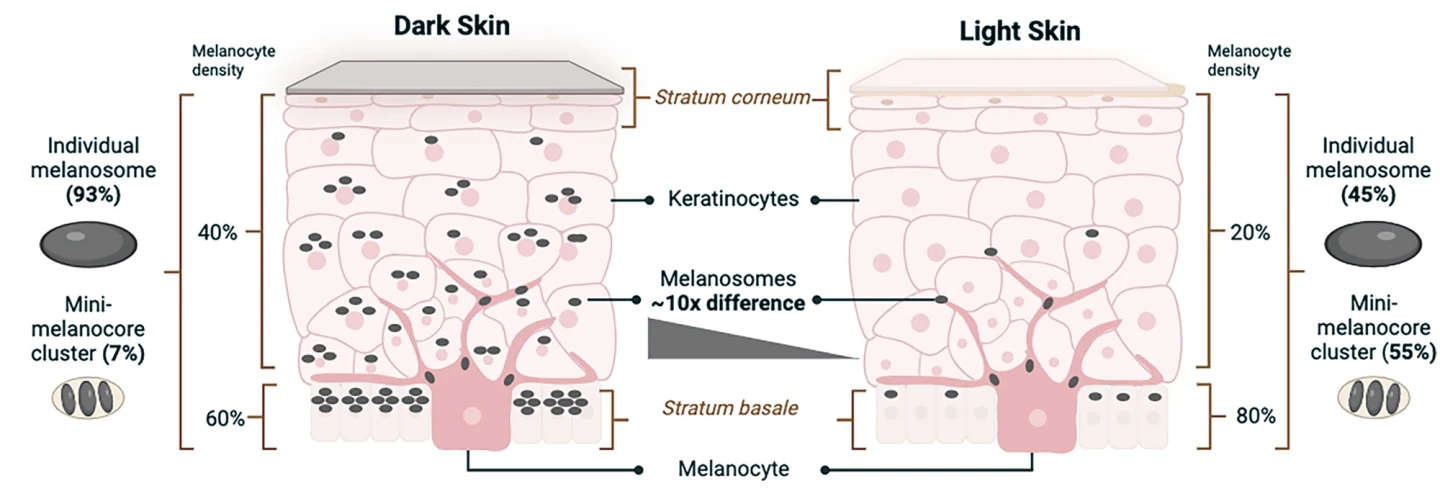
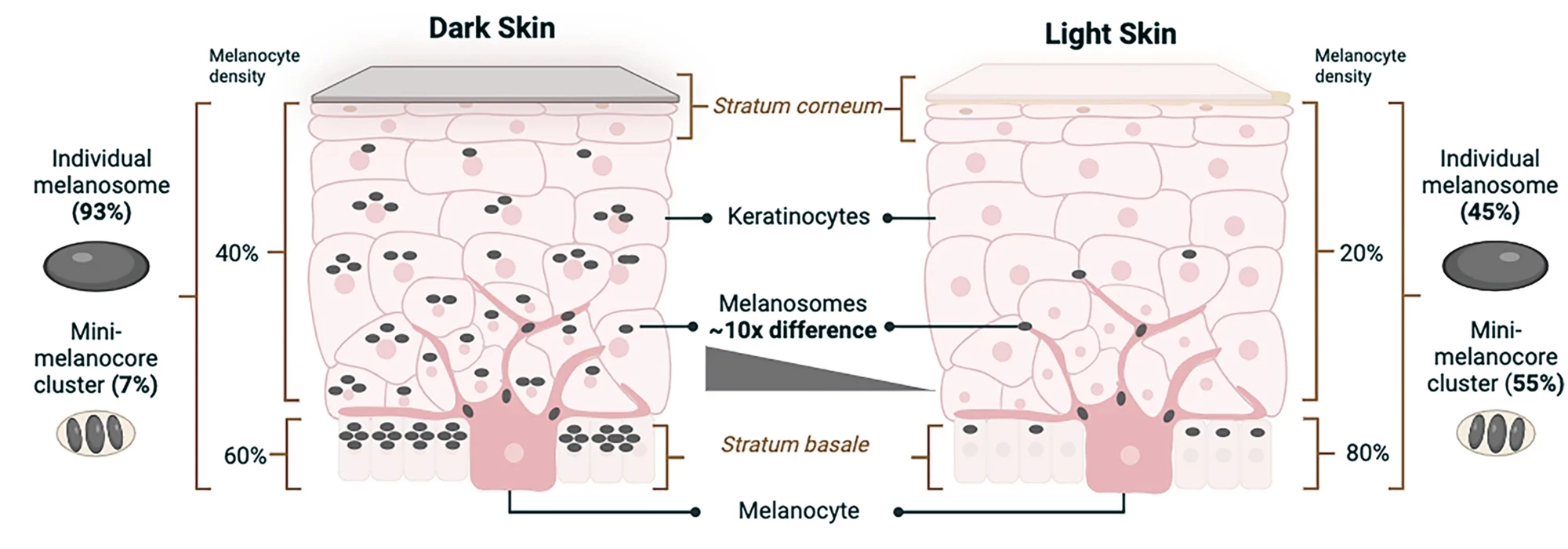
The key cells in human skin tone are melanocytes, which make melanin-containing melanosomes, and keratinocytes, which store them. The number and characteristics of melanosomes within skin layers determine variations in tone, from light to dark. Dark skin contains a higher proportion of individual large melanosomes, whereas light skin contains higher levels of clusters of mini-melanosomes. In light skin, melanosomes are concentrated in the stratum basale, the deepest skin layer, while in dark skin, they’re distributed more diffusely throughout the layers.
A person’s unique combination of the two primary forms of melanin – pheomelanin and eumelanin – is responsible for skin, hair, and eye color. Eumelanin, more than pheomelanin, plays a significant role in drug interactions due to its chemical structure, which gives it a high affinity for binding with various substances, including basic or neutrally charged drugs and metal ions. Examples of compounds with an affinity for binding with eumelanin include cocaine, nicotine, the analgesic acetaminophen, antibiotics ampicillin, ciprofloxacin, and penicillin G, antidepressants clomipramine and imipramine, and the antipsychotic medications chlorpromazine, clozapine, and haloperidol.
Let’s consider the antipsychotic clozapine: the only FDA-approved medication for treatment-resistant schizophrenia. A 2023 study used a genome-wide association study (GWAS) to examine clozapine metabolism within and between different ancestral backgrounds: European, sub-Saharan African, north African, southwest Asian, and east Asian. It found that, at the same dose, sub-Saharan African ancestry was associated with lower clozapine concentrations in the plasma compared to European ancestry.

Despite being the body’s largest organ, the skin has been largely overlooked for its potential interactions between eumelanin and drug pharmacokinetics (what the body does to a drug; how it moves into, through and out of the body) and pharmacodynamics (what a drug does to the body; the biochemical, physiologic, and molecular effects of a drug). Studies have shown that variations in skin eumelanin levels – and therefore skin tone – can influence nicotine use and dependence, which has implications for darker-skinned folks using nicotine skin patches as smoking cessation aids.
“Are we inadvertently shortchanging smokers with darker skin tones if they turn to these patches in their attempts to quit?” asked Groen.
The researchers argue that current guidelines for clinical trials fail to adequately address the impact of skin pigmentation on drug interactions.
“This oversight is particularly concerning given the push for more diverse clinical trials, as outlined in the [FDA]’s Diversity Action Plan,” Zaaijer said. “But current early-stage drug development practices still primarily focus on drug testing in white populations of Northern European descent.”
Under the provisions of the recently enacted Food and Drug Omnibus Reform Act (FDORA), the FDA requires sponsors of phase 3 clinical trials or “other pivotal studies of a drug” to submit a Diversity Action Plan designed to increase enrollment of participants from historically underrepresented populations. A ‘sponsor’ is defined as someone – an individual, pharmaceutical company, academic institution, private or other organization – who takes responsibility for and initiates a clinical investigation.
“The FDA published their draft guidelines recently,” Zaaijer said. “Once final in a few months, they will mandate considering patient diversity in clinical trials and preclinical R&D [research and development]. The next step is to provide guidance on what pharmacokinetic variables should be tested in drug R&D pipelines in their pursuit to [sic] equitable drugs.”
It's a step in the right direction, but change will take some time.
“In terms of risk profile testing, drugs are most often tested on one or a few human cell models that mostly come from donors of Northern European descent,” said Zaaijer. “Drugs are then tested in a rodent model. If these tests are successful, drug companies push the drug through to clinical trials. But are drugs ready to be given to a diverse patient group if they haven’t first been tested, for example, on human cell models of different ancestries? Would you bungee jump off a bridge if you know the ropes have not been tested for your weight category? Unlikely. So why is this currently acceptable with drugs?”
As a fix, Groen and Zaaijer have proposed that pharmaceutical companies use differently pigmented 3D human skin models to assess the binding properties of new drugs across different skin tones.
“Skin pigmentation should be considered as a factor in safety and dosing estimates,” said Zaaijer. “We stand on the brink of a transformative era in the biomedical industry, where embracing inclusivity is not just an option anymore but a necessity.”
The researchers also encourage patients and clinical trial participants to ask questions like, ‘Has this drug been tested to see if it’s safe for people from different ancestral backgrounds, like mine?’
“If different ancestral backgrounds are taken into consideration in the early stages of drug discovery, then diverse groups of people may have more trust in the drug development process and enroll in clinical trials because they will be better informed of any potential associated risks,” said Groen.
The perspective article was published in the journal Human Genomics.
Source: UC Riverside