Researchers have transplanted precursor stem cells into the damaged heart muscle of pigs, repairing injured cells and improving heart function. The study may lead to a treatment that can regenerate heart muscle damaged by a lack of oxygen.
Cardiac ischemia occurs when the heart muscle is starved of oxygen. If it’s prolonged, ischemia can cause irreversible damage and heart failure, affecting the heart’s ability to pump effectively. The most common cause of cardiac ischemia is atherosclerosis, a buildup of plaques in the arteries. If an artery is blocked completely by a plaque, it results in a heart attack or myocardial infarction.
Previous studies have looked at ways of reversing the heart muscle damage caused by ischemia, including transplanting human pluripotent stem cells (hPSCs), which are are immature cells that can renew themselves by dividing and differentiating into primary groups of cells that make up the human body. They can be used to create any cell or tissue needed.
In preclinical trials, researchers from the Duke-National University of Singapore (Duke-NUS) Medical School grew lab-made hPSCs and caused them to differentiate into precursor heart muscle cells called cardiac progenitor cells. Key to the process was the researchers’ use of laminin, a protein that directs the development of certain tissue cell types. Here, the researchers grew the progenitor cells on the type of laminin found in the heart.
Approximately 200 million 11-day-old progenitor cells were injected into the damaged heart muscle of pigs. They were seen to rapidly organize themselves in the damaged tissue, generate a heart muscle graft and continue to mature.
“As early as four weeks after the injection, there was rapid engraftment, which means the body is accepting the transplanted stem cells,” said Lynn Yap, lead author of the study. “We also observed the growth of new heart tissue and an increase in functional development, suggesting that our protocol has the potential to be developed into an effective and safe means for cell therapy.”
The researchers also saw a significant improvement in the heart’s ability to pump and a reduction in the size of the area of muscle death caused by ischemia.
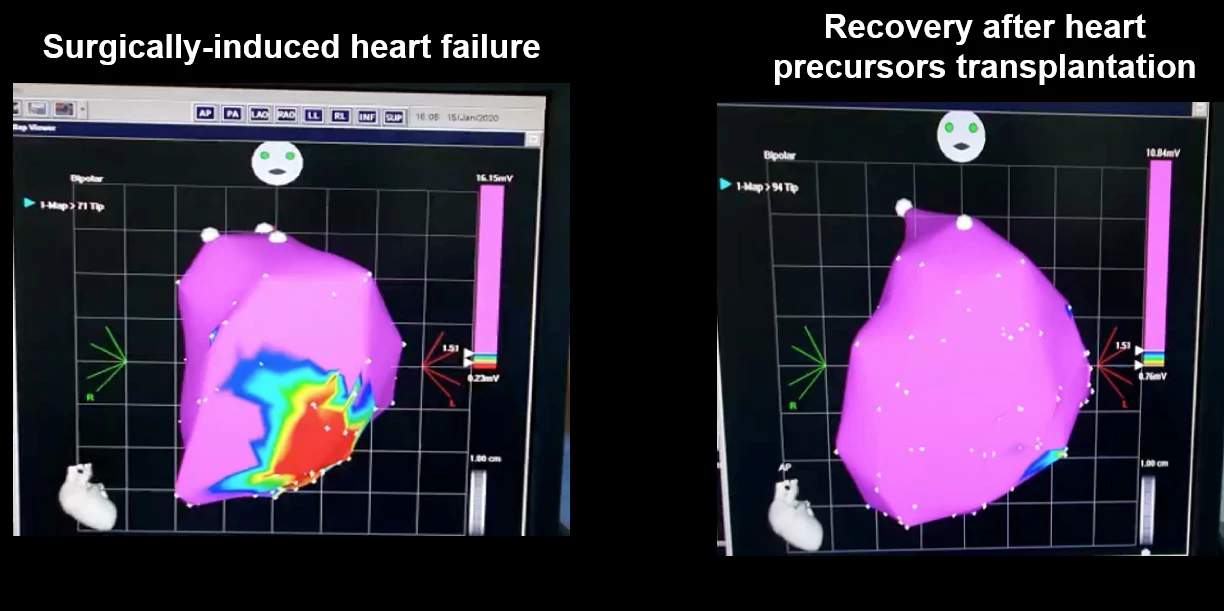
Previous studies transplanted heart muscle cells that had already started beating, which led to fatal heart arrhythmias. In the current study, the researchers used non-beating cells that matured and started beating after transplantation. Using non-beating heart cells reduced the incidence of arrhythmia by half. When arrhythmias did occur, they were temporary and resolved themselves in around 30 days. Additionally, the transplanted cells did not trigger tumor formation, another concern related to stem cell therapies.
The researchers say their technique is easily reproducible – and safe – thanks to using laminin to grow stem cells.
“To ensure patient safety, it is imperative that cell-based therapies show consistent efficacy and reproducible results,” said Enrico Petretto, one of the study’s co-authors. “By extensive molecular and gene expression analysis, we demonstrated that our laminin-based protocol for generating functional cells to treat heart disease is highly reproducible.”
The study’s promising results may lead to a treatment that can regenerate heart muscle damaged by ischemia.
“Our technology brings us one step closer to offering a new treatment for heart failure patients, who would otherwise live with diseased hearts and have slim chances of recovery,” said Karl Tryggvason, a corresponding author of the study. “It will also have a major impact in the field of regenerative cardiology by offering a tried-and-tested protocol that can restore damaged heart muscles while reducing the risk of adverse side effects.”
The study was published in the journal NPJ Regenerative Medicine.





