Drug resistance is an increasing problem in bacterial infections, but a new study may have uncovered a way to reinvigorate old antibiotics. Scientists have wrapped gold nanoparticles in molecules that seek out bacteria and disrupt their cell membranes, allowing existing drugs to kill them more easily.
It’s a perfect example of evolution in action – using antibiotics subjects bacteria to environmental pressures that means only the toughest survive. Eventually, the resistance genes spread so wide that the drug no longer has any effect on that species. That forces us to use other drugs, and the pattern repeats. Now, with our last line of defense beginning to fail and predictions of millions of deaths per year by 2050, it looks like we’re losing that arms race.
Along with developing new antibiotics, scientists are looking to find ways to get old ones working again. That includes modifying drugs, pairing them up with other antibiotics or probiotics, or using peptides to weaken the bugs first. The new discovery works a little like that last method.
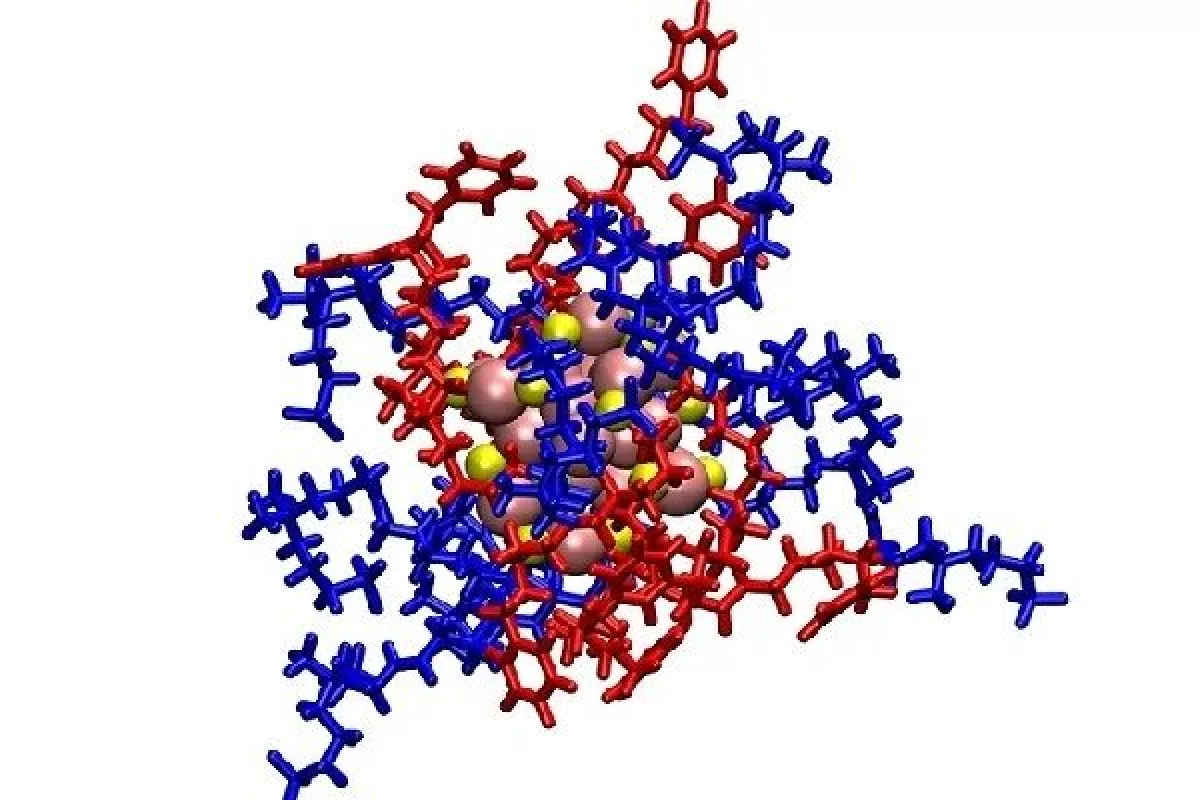
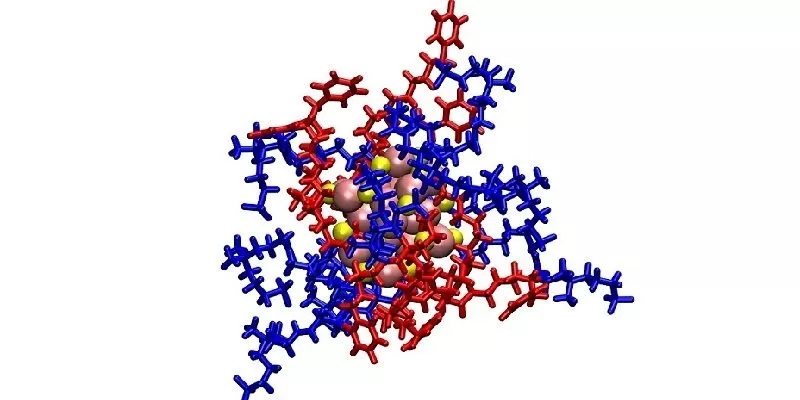
Researchers from the University of Leeds, the Southern University of Science and Technology and Fudan University created a new antibacterial weapon out of a few established pieces. The core is a nanocluster of around 25 atoms of gold, which has long been investigated for its antimicrobial properties.
The gold nanoclusters were packaged inside a molecular envelope that helped them reach bacteria without harming the host cells. This envelope was made of two ligands – the first has a positive charge that’s attracted to the negative charge of the bacteria, while the second is what’s known as a zwitterionic group, which contains both positive and negative charges. Since these are also found in the membranes of mammal cells, this helps the package ignore the host. Once its job is done, this also helps it pass through the kidneys and out of the body via the urine.
The idea is that these ligands together would help deliver the package to the bacteria, where the gold nanocluster would then get to work disrupting the bugs’ membranes. That might not be enough to kill them directly, but it should weaken them enough to allow antibiotics to finish them off – even if the bacteria had already developed resistance to them.
The team tested the technique in mice on a common hospital-acquired superbug called methicillin resistant Staphylococcus epidermidis (MRSE). They treated the bacteria with three antibiotics from three different classes, both with and without the gold nanoclusters. And sure enough, those paired with the gold performed much better, inhibiting the MRSE using just one-128th the amount of drug needed when given alone.
The technique could help buy some time to develop new antibiotics, by breathing fresh life into old ones.
The research was published in the journal Chemical Science.
Source: University of Leeds