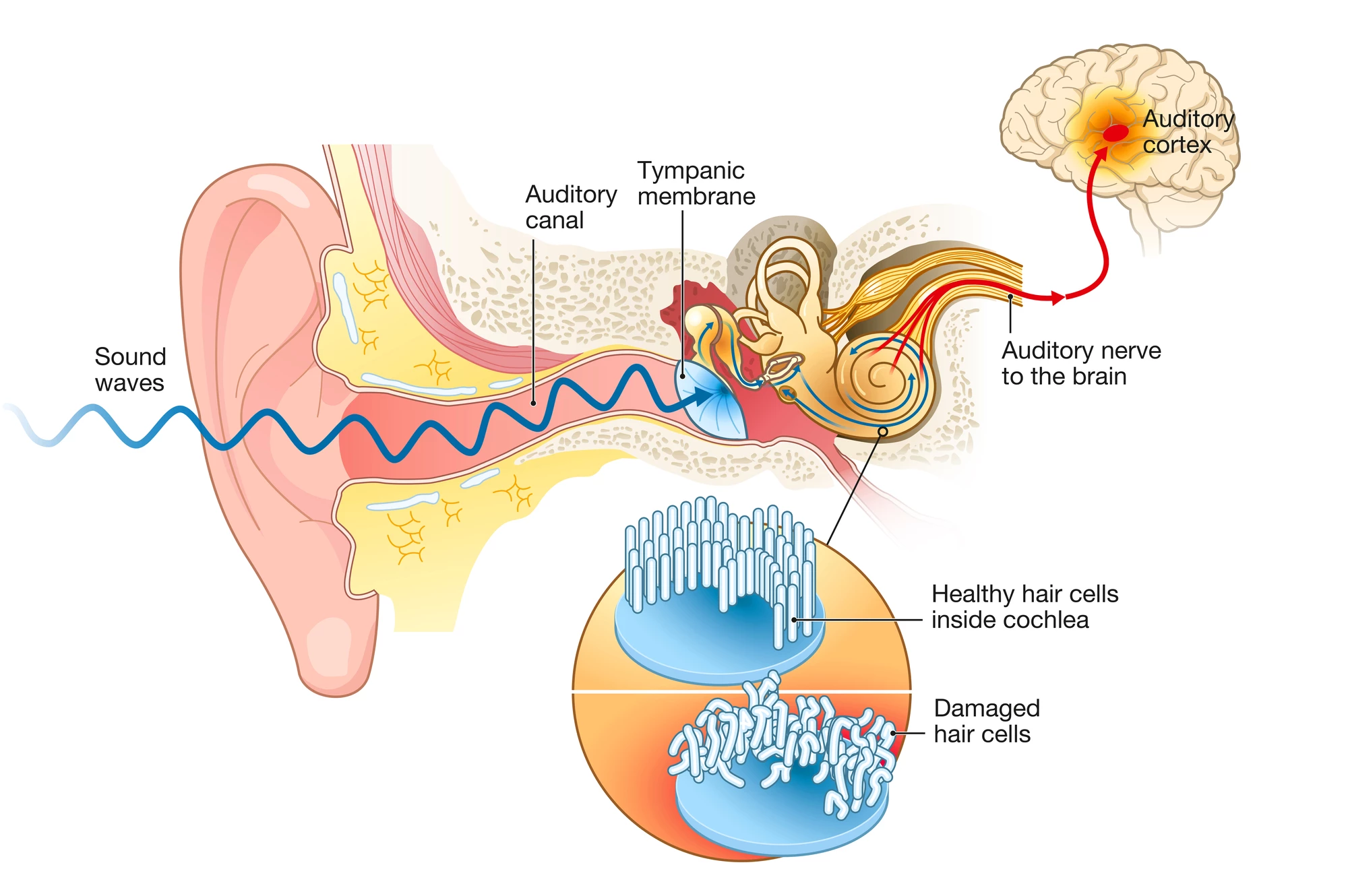
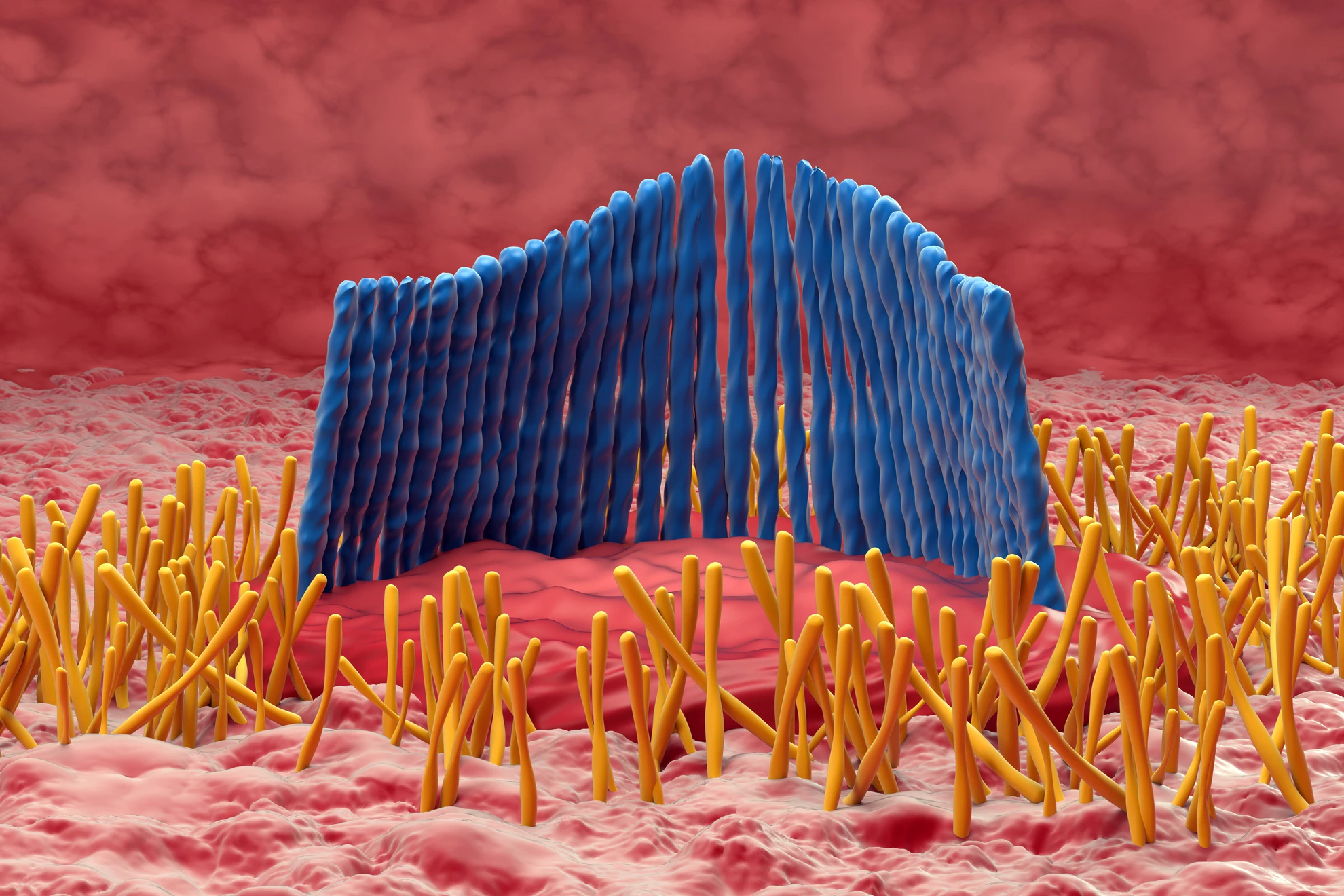
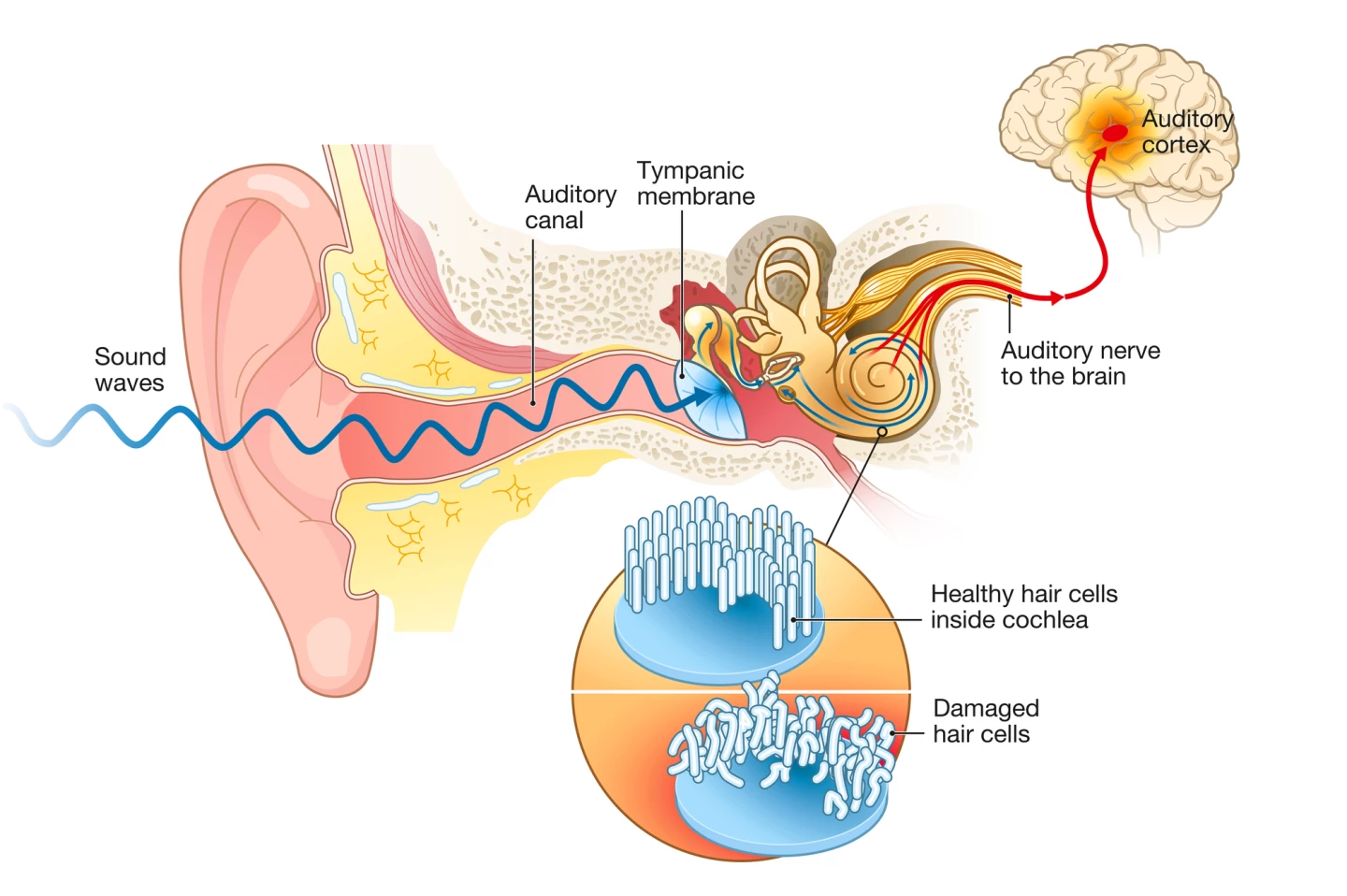
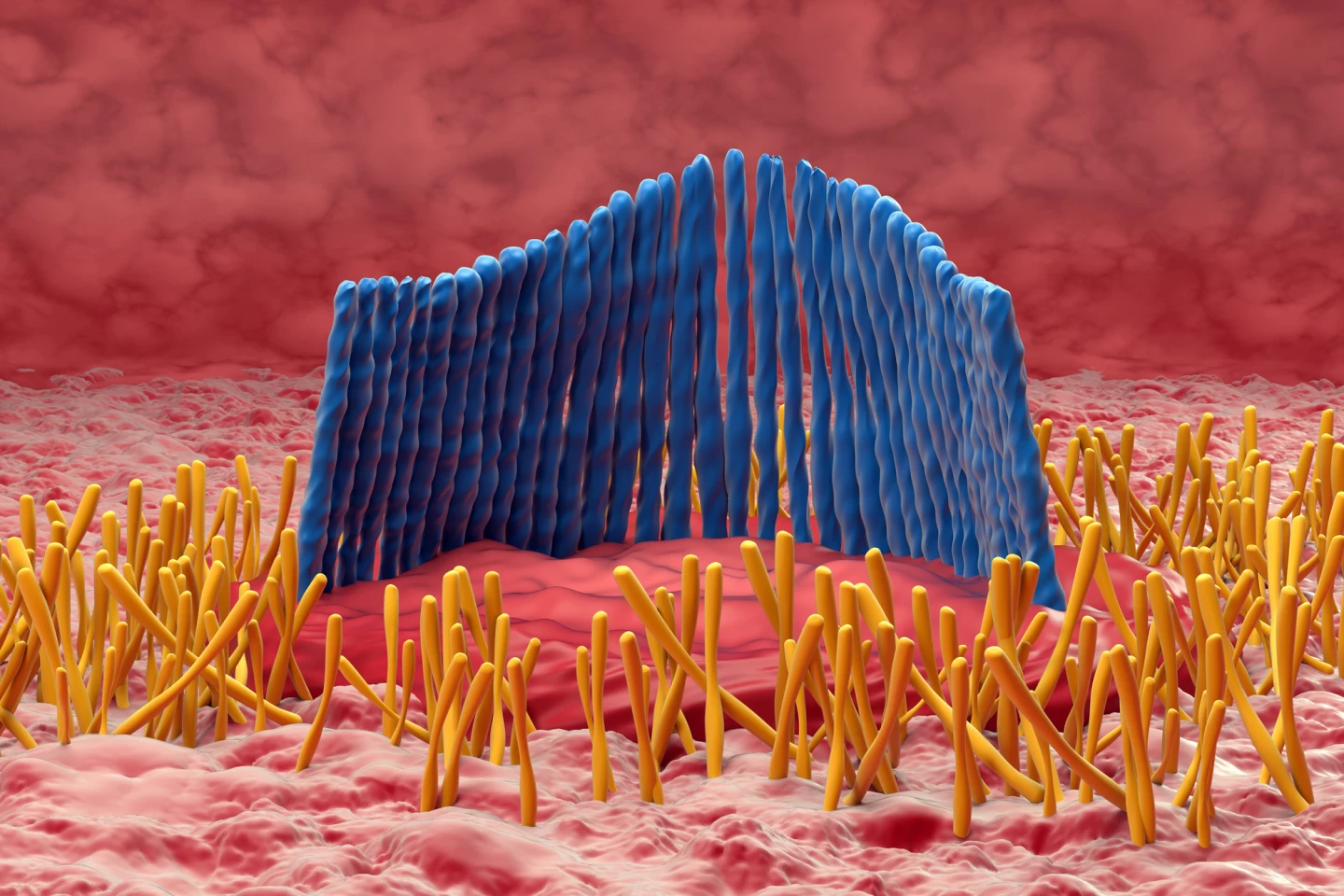
Age-related hearing loss was primarily thought to be caused by damage to or destruction of hair cells. A row of inner hair cells in the snail-like cochlea converts sound vibrations into electrical signals that are transmitted to the brain via the auditory nerve. However, recent research suggests that damage to the synapses that connect these hair cells to auditory nerve fibers may be the first step in the process of hearing loss.
A new study by researchers at Michigan Medicine’s Kresge Hearing Research Institute found that tweaking the expression of a particular protein to increase the number of synapses improved auditory processing to better-than-normal levels in otherwise healthy mice.
Auditory processing is different to hearing. Whereas hearing is the conversion of sound waves to auditory signals that are sent to the brain, auditory processing is what the brain does with the auditory information it receives from the ears.
The researchers had previously experimented with neurotrophin-3 (Ntf3), a protein that controls the survival and differentiation of neurons in mammals. A 2014 study found that an overproduction of Ntf3 led to more synapses and boosted their regeneration following exposure to two hours of 100-dB noise. Then, in 2022, a study found that higher levels of Ntf3 improved age-related hearing loss in middle-aged mice.
“We knew that providing Ntf3 to the inner ear in young mice increased the number of synapses between inner hair cells and auditory neurons, but we did not know what having more synapses would do to hearing,” said Gabriel Corfas, director of the Kresge Institute and corresponding author on this and the previous two studies. “We now show that animals with extra inner ear synapses have normal thresholds – what an audiologist would define as normal hearing – but they can process the auditory information in supranormal ways.”
As they’d done in previous studies, the researchers altered the expression of Nft3 in mice to produce two groups: one with more synapses and one with fewer. Both groups underwent a Gap-Prepulse Inhibition of the Acoustic Startle Reflex (GPIAS) test to measure auditory temporal processing or the perception of sound within a restricted or defined time. In humans, results of the GPIAS test have been shown to correlate with auditory processing.
The test involves placing a mouse in a chamber with background noise. A loud tone that startles the mouse is either played alone or preceded by a very brief silent gap. When the mouse detects the gap, the startle response is reduced (it figures out that it’s coming because of the silence). When they measured how long the silent gap needed to be for the mice to detect it, the researchers found that those with fewer synapses required a much longer silent gap. The finding suggests that having fewer synapses causes a deficit in auditory processing.
Less expected was the finding that mice with an increased number of synapses performed better on the GPIAS test and on the Auditory Brainstem Response (ABR) test, which measures how well the cochlea and the brain’s hearing pathways are working. Both suggest an ability to process an increased amount of auditory information.
“We were surprised to find that when we increased the number of synapses, the brain was able to process the extra auditory information,” Corfas said. “And those subjects performed better than the control mice in the behavioral test. This suggests that this molecule has the potential to improve hearing in humans in similar situations. The new results indicate the regenerating synapses or increasing their numbers will improve their auditory processing.”
The researchers say that their findings could lead to new treatments for some hearing disorders, as well as other conditions.
“Some neurodegenerative disorders also start with loss of synapses in the brain,” said Corfas. “Therefore, the lessons from the studies in the inner ear could help in findings new therapies for some of these devastating diseases.”
The study was published in the journal PLOS Biology.
Source: Michigan Medicine