It makes up more than half of the human body and covers around 70 percent of the Earth's surface, but there is still a lot we don't understand about water. By using cutting-edge tools to study microscopic jets of the liquid, scientists have spotted water molecules pushing and pulling on each other in what is described as a "quantum tug," a phenomenon that has never been directly observed before and might help explain some of water's strange behavior.
The groundbreaking research was carried out by scientists at Stanford University and Stockholm University, who set out to explore the forces that hold water molecules together. Within any water molecule is one oxygen atom and two hydrogen atoms. The positively charged hydrogen atoms in one molecule are attracted to the negatively charged oxygen atoms in another, which has the effect of binding the molecules together.
Water has a raft of strange properties that set it apart from many other liquids, and defy many of the rules around what liquids should do. A solid slab of butter wouldn't float atop a bath of melted butter, but drop a few ice cubes into a glass of water and that's exactly what that solid water will do. Water also offers an incredibly high surface tension that enables insects to walk across its surface, and it has a good ability to store heat and help keep ocean temperatures stable.
Scientists believe that a better understanding of hydrogen bonds between molecules could help explain these sorts of strange properties, and the authors of the new study have taken an important step toward that objective.
“For a long time, researchers have been trying to understand the hydrogen bond network using spectroscopy techniques,” says leader of the study, Jie Yang. “The beauty of this experiment is that for the first time we were able to directly observe how these molecules move.”

The reason this phenomenon has been so difficult to observe is because the movements of the hydrogen bonds are very fast and very subtle. But by deploying a high-speed "electron camera," which uses a powerful beam of electrons that scatter away from a subject to reveal very fine molecular movements, the scientists were able to capture the hydrogen bonds in the act.
The experiments involved very fine jets of water measuring 100 nanometers thick, or around 1,000 times thinner than a human hair, which were blasted with infrared light that caused the water molecules to vibrate. The electron camera was then used to generate high-resolution snapshots of the shifting atoms within the water molecules, which told a story of how they interact.
As the excited water molecules began to vibrate, the hydrogen atoms began to tug at oxygen atoms in neighboring molecules, pulling them closer and then repelling them away with even greater strength. Described as a "quantum tug," this action wound up expanding the space between the molecules.
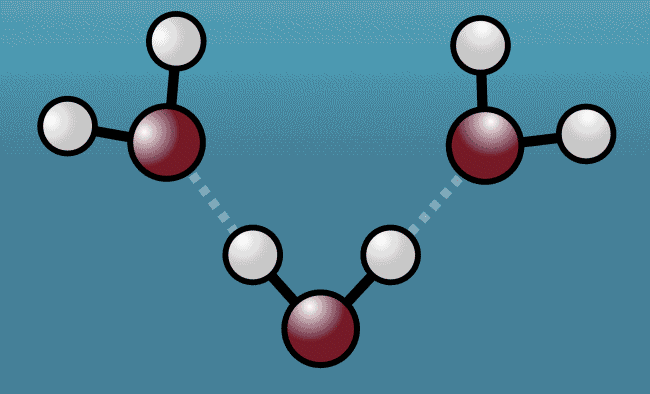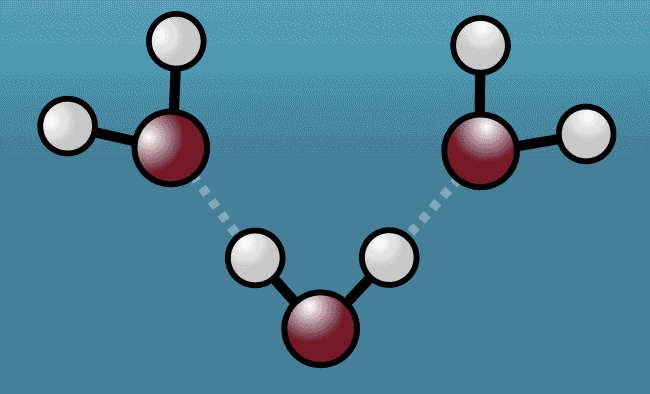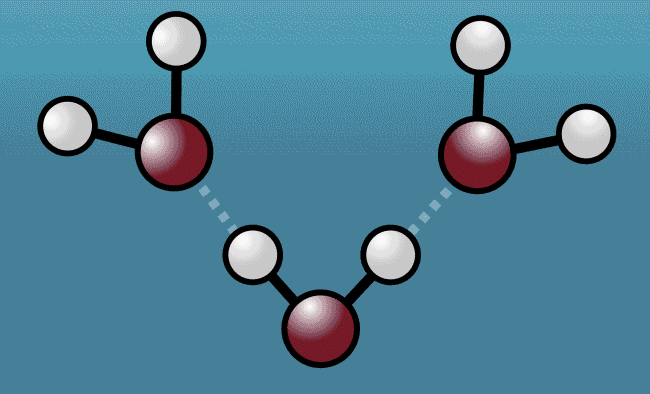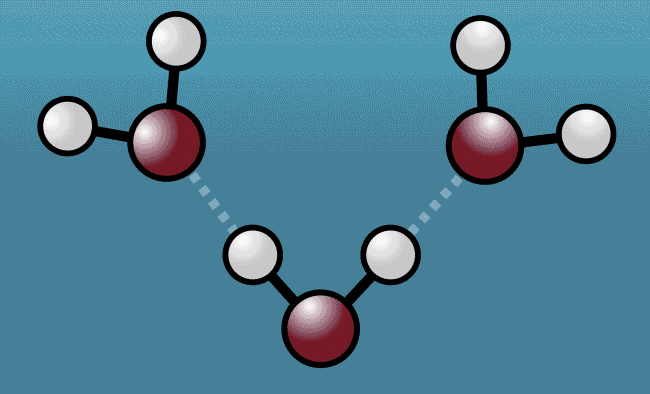
“Although this so-called nuclear quantum effect has been hypothesized to be at the heart of many of water’s strange properties, this experiment marks the first time it was ever observed directly,” says study collaborator Anders Nilsson. “The question is if this quantum effect could be the missing link in theoretical models describing the anomalous properties of water.”
The scientists hope to continue using this method to better understand water's unusual properties. This could lead to an improved understanding of not just water itself, but the role it plays in living organisms.
“This has really opened a new window to study water,” says Xijie Wang, study collaborator. “Now that we can finally see the hydrogen bonds moving, we’d like to connect those movements with the broader picture, which could shed light on how water led to the origin and survival of life on Earth and inform the development of renewable energy methods.”
The research was published in the journal Nature.
Source: Stanford University





