Gut support cells communicate with surprising precision – like brain neurons – using tiny extensions to send instructions to the stem cells responsible for maintaining and healing the intestine. The discovery may change our understanding of tissue repair and gut diseases.
Our intestinal lining, or epithelium, rapidly and continually renews itself, replacing its entire cell population every four to five days. Stem cells, which live in tube-like "crypts" deep in the gut lining, are vital to the renewal process, dividing and differentiating into different cell types that replace old cells.
New research by Duke-NUS Medical School and Nanyang Technological University, Singapore (NTU Singapore), has uncovered an unexpected – and precise – communication system that exists in the gut, similar to the way neurons signal one another in the brain.
“Sometimes, when you study the basics closely, you uncover something transformative,” said principal research scientist Dr Gediminas Greicius, from Duke-NUS’ Cancer and Stem Cell Biology (CSCB) Program and the lead and corresponding author of the study. “This system of targeted signaling was hiding in plain sight, and now that we see it, it reshapes our understanding of the biology of stem cells in the gut.”
It comes down to Wnts, signaling molecules that help control the behavior of intestinal stem cells located in the crypts of the intestine. Actually, they’re located within a "niche" within a crypt, a highly regulated microenvironment that supports and regulates their behavior. When Wnt signaling is activated, it promotes the growth and division of these stem cells. Wnt signals help maintain a balance between stem cell self-renewal (keeping enough stem cells in the crypts) and differentiation (turning some stem cells into specialized cells that make up the lining of the intestine).
Initially, it was thought that Wnts simply diffused through the tissue, eventually reaching their stem cell targets. However, the present study overturned that hypothesis.
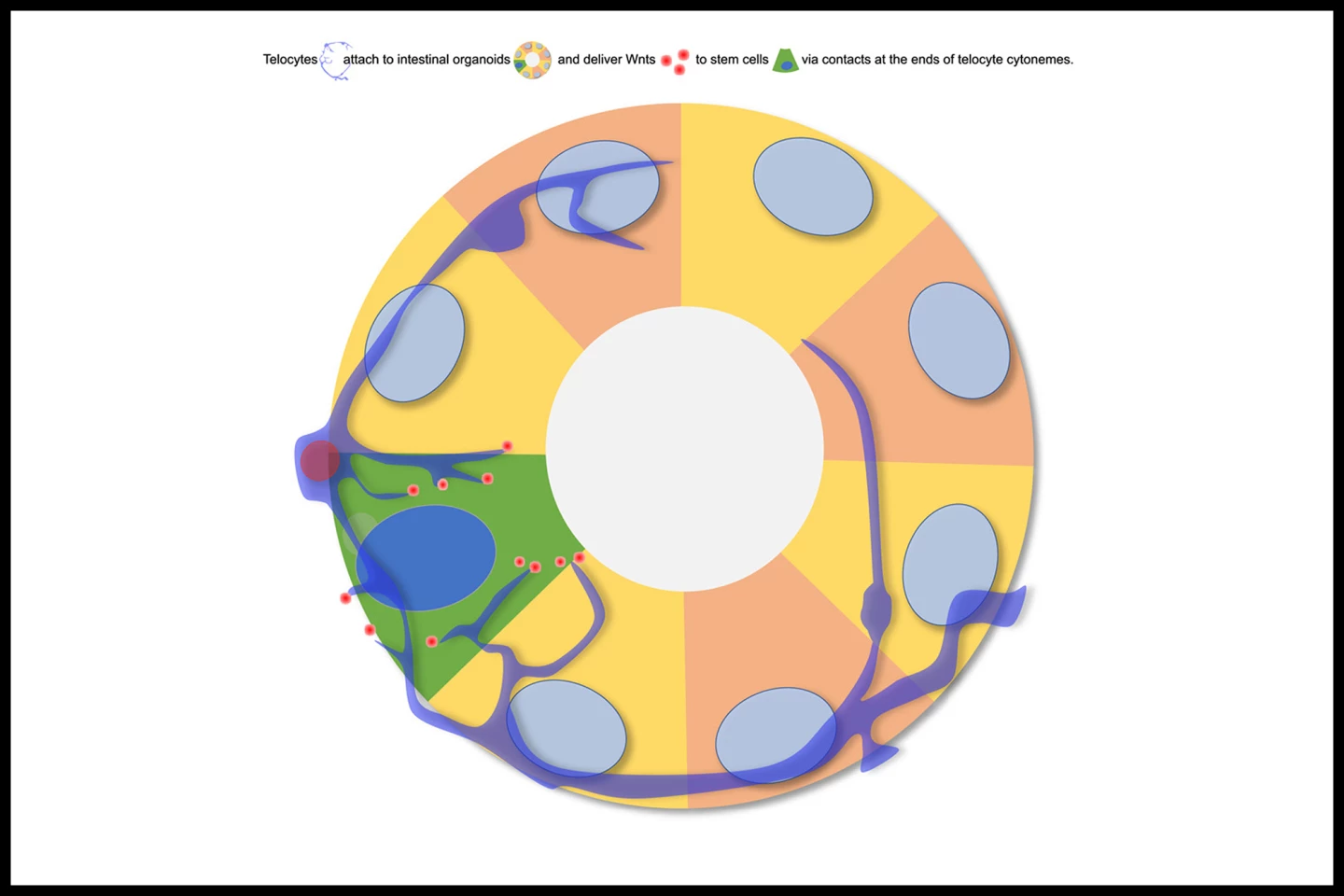
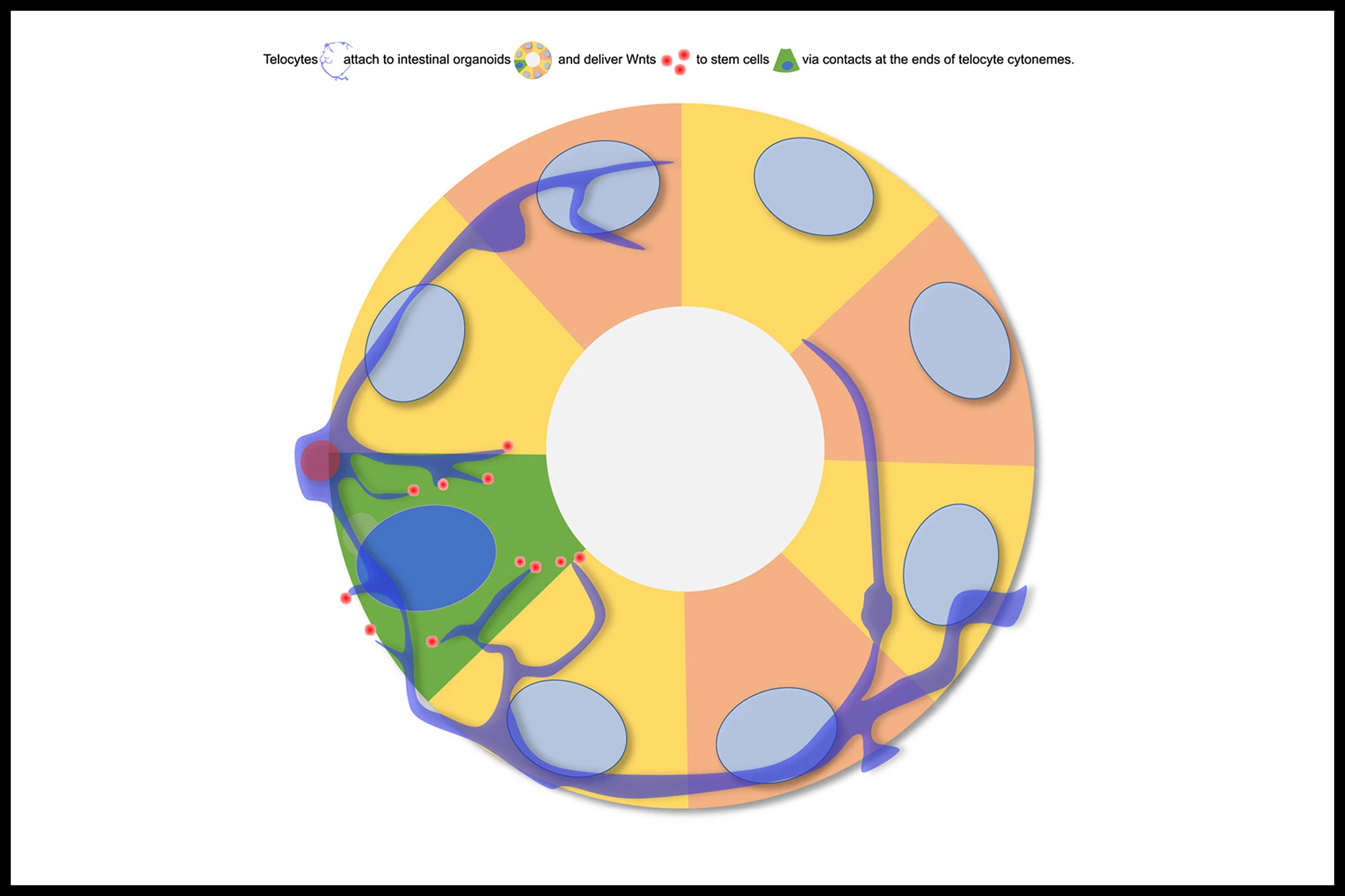
“We discovered that these signals aren’t just drifting through tissue,” said co-corresponding author Professor David Virshup, a cancer biologist and the Director of the CSCB. “They’re being delivered with surprising precision from the niche to the stem cells by specialized cells or telocytes – changing the way we think about cellular communication in the gut, similar to how neurons pass signals to one another in the brain.”
The interesting thing about telocytes is their ability to form long, thin filamentous extensions called cytonemes that reach directly from the telocyte to the stem cell. Using advanced imaging techniques, the researchers observed that, in a mouse intestine, the telocytes use cytonemes to deliver Wnts directly to individual stem cells within the crypt. They also observed that the telocyte-stem cell contact points resemble synapses, the specialized junctions where neurons communicate, underscoring the brain-like precision of this system.
“This kind of direct, cell-to-cell communication highlights a new level of precision in how secreted molecules are delivered to their target cell,” said Assistant Professor Alexander Ludwig from the School of Biological Sciences at NTU Singapore. “It’s a striking example of how imaging at different scales coupled with new protein tagging approaches can uncover novel mechanisms and change paradigms.”
The implications of this study’s findings are potentially far-reaching. It’s already known that disruptions in Wnt signaling can drive some colon cancers and might play a role in chronic inflammatory bowel disease (IBD) such as Crohn’s disease and ulcerative colitis.
“This discovery could change how we approach tissue repair and regenerative medicine,” said Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, who was not involved in the study. “If we can harness or restore this precise mode of signaling, it may enhance the effectiveness of stem cell therapies and help develop more targeted treatments for gut-related diseases. It’s a strong example of how basic science drives real-world impact.”
The study was published in the journal Developmental Cell.
Source: Duke-NUS Medical School